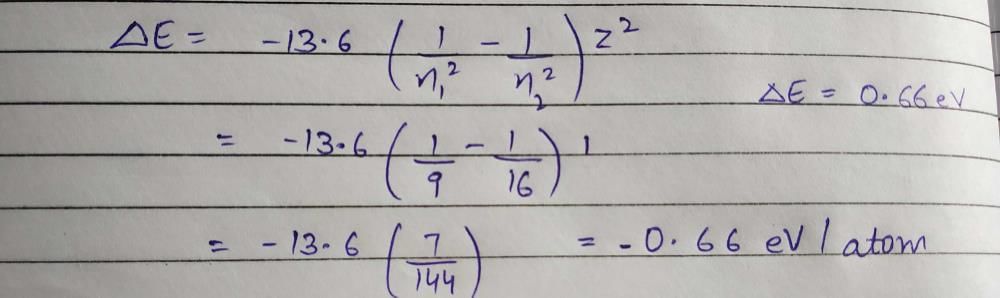All Exams >
NEET >
Topic-wise MCQ Tests for NEET >
All Questions
All questions of Atoms for NEET Exam
Number of alpha particles N scattered at an angle θ during Rutherford’s alpha scattering experiment is :- a)
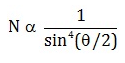
- b)
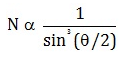
- c)
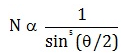
- d)
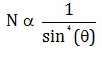
Correct answer is option 'A'. Can you explain this answer?
Number of alpha particles N scattered at an angle θ during Rutherford’s alpha scattering experiment is :
a)
b)
c)
d)

|
Sushil Kumar answered |
Answer :- a
Solution :- For a single scatterer, such as a single gold nucleus within a thin gold foil layer, the differential scattering cross section is defined as follows [2]:
dσ(θ, φ)/dΩ = flux scattered into element dΩ at angles (θ, φ)/incident flux per unit area
dσ/dΩ = (Iθ × A)/ (dΩ × I0 × NAvo × ρ × x(foil))........... (1)
where NAvo is Avogadro’s number, xfoil is the thickness of the target foil, A is the atomic mass of the material in the target foil, dΩ is the solid-angle of the detector, I0 is the unattenuated intensity of the alpha particle beam.
dσ/dΩ = [(ZZ0 e^2/4E )^2]/(1/sin^4(θ/2 ))
dσ/dΩ is directly proportional to 1/sin^4(θ/2 )
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
- a)Statement i
- b)Statement iv
- c)Statement ii
- d)Statement iii
Correct answer is option 'C'. Can you explain this answer?
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
a)
Statement i
b)
Statement iv
c)
Statement ii
d)
Statement iii
|
|
Hansa Sharma answered |
Statement i. Radius of Bohr's orbit of hydrogen atom is given by
r= n2h2/4π2mKze2
or, r=(0.59A˚)(n2/z)
So, from expression we found r∝n2
Hence the 1st statement is correct.
Statement ii.
We know that
En=-13.6 x z2/n2
So, En ∝1/n2
Hence the 2nd statement is wrong.
Statement iii.Bohr defined these stable orbits in his second postulate. According to this postulate:
r= n2h2/4π2mKze2
or, r=(0.59A˚)(n2/z)
So, from expression we found r∝n2
Hence the 1st statement is correct.
Statement ii.
We know that
En=-13.6 x z2/n2
So, En ∝1/n2
Hence the 2nd statement is wrong.
Statement iii.Bohr defined these stable orbits in his second postulate. According to this postulate:
- An electron revolves around the nucleus in orbits
- The angular momentum of revolution is an integral multiple of h/2π – where Planck’s constant [h = 6.6 x 10-34 J-s].
- Hence, the angular momentum (L) of the orbiting electron is: L = nh/2 π
Hence the 3rd statement is correct.
Statement iv.According to Bohr's theory
Angular momentum of electron in an orbit will be Integral multiple of (h/2π)
Magnitude of potential energy is twice of kinetic energy of electron in an orbit
∣P.E∣=2∣K.E∣
K.E=(13.6ev)( z2/n2)
Hence, The 4th statement is correct.
Statement iv.According to Bohr's theory
Angular momentum of electron in an orbit will be Integral multiple of (h/2π)
Magnitude of potential energy is twice of kinetic energy of electron in an orbit
∣P.E∣=2∣K.E∣
K.E=(13.6ev)( z2/n2)
Hence, The 4th statement is correct.
Rutherford’s experiments on scattering of alpha particles proved that:- a)atoms contain electrons
- b)number of positive charges is equal to the number of negative charges
- c)atom is mostly empty
- d)positive charge is uniformly distributed in the atom
Correct answer is option 'C'. Can you explain this answer?
Rutherford’s experiments on scattering of alpha particles proved that:
a)
atoms contain electrons
b)
number of positive charges is equal to the number of negative charges
c)
atom is mostly empty
d)
positive charge is uniformly distributed in the atom

|
Divey Sethi answered |
Most of the α-particle passed through the foil straight without suffering any change in their direction. This shows that most of the space inside the atom is empty or hollow.
A small fraction of α-particles was deflected through small angles and a few through larger angles. For this to happen α- particles (positively charged) must approach a heavy positively charged core inside the atom (like charges repel each other). This heavy positively charged core inside the atom was named as the nucleus.
A small fraction of α-particles was deflected through small angles and a few through larger angles. For this to happen α- particles (positively charged) must approach a heavy positively charged core inside the atom (like charges repel each other). This heavy positively charged core inside the atom was named as the nucleus.
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by- a)
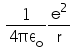
- b)
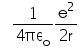
- c)
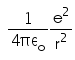
- d)
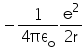
Correct answer is option 'B'. Can you explain this answer?
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by
a)
b)
c)
d)
|
|
Swati Verma answered |
K.E. of nth orbit
=> (1/k) Ze2/2r
For H atom,
K.E.=(1/4πε) x (e2/2r)
=> (1/k) Ze2/2r
For H atom,
K.E.=(1/4πε) x (e2/2r)
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?- a)All the alpha particles would have been deflected by the foil.
- b)All the alpha particles should have bounced straight back from the foil.
- c)Alpha particles should have passed through the foil with little or no deflection.
- d)Alpha particles should have become embedded in the foil.
Correct answer is option 'C'. Can you explain this answer?
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?
a)
All the alpha particles would have been deflected by the foil.
b)
All the alpha particles should have bounced straight back from the foil.
c)
Alpha particles should have passed through the foil with little or no deflection.
d)
Alpha particles should have become embedded in the foil.

|
Snehal Gosavi answered |
Correct Option C ===>ΔΔΔ
ΔAlfa particles are massive particles and they have speed when they bombarded....
ΔAccording plum pudding model if atom given by Thomson protons and electrons are equally distributed....
Δthat's why massive alpha particles will pass through the foil with little deflection (due to protons)......
ΔBcoz here protons are like spreaded cloud and not in nucleus ( massive part or atom) ....
Δand alpha particle is more massive than single proton....so they will not deflect due to protons and will pass through foil....
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:- a)the deflected angle is always large
- b)they are usually observed with kinetic energy between 5 and 10 MeV
- c)some alpha particles are deflected to large angles
- d)the deflected angle is usually small
Correct answer is option 'C'. Can you explain this answer?
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:
a)
the deflected angle is always large
b)
they are usually observed with kinetic energy between 5 and 10 MeV
c)
some alpha particles are deflected to large angles
d)
the deflected angle is usually small
|
|
Tanuja Kapoor answered |
In ruther ford experiment he suggest that all the positive charge and mass are concentrated at the centre when he bombarded the alpha partical which is dipositive in nature and when it is more close to centre it get deflect to a large angle and with increase of closenes to centre its deflection angle increase and some alpha partical deflect to 180 degree so it prove that all the positive charge and mass are concentrated at the centre where as acccording to thomson atom is hard solid sphere in which its total +ve charge and mass uniformalyy distributed on the surface and electrone reside as seed in watermelon ( plum pudding model)
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because- a)they produce more white light
- b)they do not use uv radiations
- c)they do not waste as much energy producing (invisible) infrared photons
- d)they do not waste as much energy producing visible photons
Correct answer is option 'C'. Can you explain this answer?
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because
a)
they produce more white light
b)
they do not use uv radiations
c)
they do not waste as much energy producing (invisible) infrared photons
d)
they do not waste as much energy producing visible photons
|
|
Riya Banerjee answered |
The phosphor fluoresces to produce light. A fluorescent bulb produces less heat, so it is much more efficient. This makes fluorescent bulbs four to six times more efficient than incandescent bulbs. That's why you can buy a 15-watt fluorescent bulb that produces the same amount of light as a 60-watt incandescent bulb.
Which of these is true?- a)The alpha particles used in Rutherford’s experiment are positively charged particles
- b)Dalton assumed that atoms are made up of electrons, protons, and neutrons
- c)In Rutherford’s alpha scattering experiment, all of the alpha particles passed through the gold foil.
- d)JJ Thomson determined the charge and mass of electrons
Correct answer is 'A'. Can you explain this answer?
Which of these is true?
a)
The alpha particles used in Rutherford’s experiment are positively charged particles
b)
Dalton assumed that atoms are made up of electrons, protons, and neutrons
c)
In Rutherford’s alpha scattering experiment, all of the alpha particles passed through the gold foil.
d)
JJ Thomson determined the charge and mass of electrons
|
|
Shalini Basu answered |
**Explanation:**
The correct answer is **a) The alpha particles used in Rutherford's experiment are positively charged particles**.
Rutherford's experiment, also known as the gold foil experiment, was conducted in 1909 by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden. The experiment aimed to understand the structure of the atom and investigate the distribution of positive charge within it.
In this experiment, Rutherford and his team bombarded a thin gold foil with a beam of alpha particles. Alpha particles are positively charged particles that consist of two protons and two neutrons, which are the same as helium nuclei. They are emitted from a radioactive source, such as radium or polonium.
Rutherford observed that while most of the alpha particles passed straight through the gold foil, some of them were deflected at different angles, and a very small fraction bounced back in the direction opposite to the source. This unexpected result led to the discovery of the atomic nucleus and the concept of a mostly empty space within the atom.
Based on the observations from the experiment, Rutherford proposed a new atomic model known as the nuclear model. According to this model, atoms have a dense, positively charged nucleus at the center, which contains most of the atom's mass. The electrons, which are negatively charged particles, orbit around the nucleus in specific energy levels.
Therefore, the correct answer is a) The alpha particles used in Rutherford's experiment are positively charged particles.
The correct answer is **a) The alpha particles used in Rutherford's experiment are positively charged particles**.
Rutherford's experiment, also known as the gold foil experiment, was conducted in 1909 by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden. The experiment aimed to understand the structure of the atom and investigate the distribution of positive charge within it.
In this experiment, Rutherford and his team bombarded a thin gold foil with a beam of alpha particles. Alpha particles are positively charged particles that consist of two protons and two neutrons, which are the same as helium nuclei. They are emitted from a radioactive source, such as radium or polonium.
Rutherford observed that while most of the alpha particles passed straight through the gold foil, some of them were deflected at different angles, and a very small fraction bounced back in the direction opposite to the source. This unexpected result led to the discovery of the atomic nucleus and the concept of a mostly empty space within the atom.
Based on the observations from the experiment, Rutherford proposed a new atomic model known as the nuclear model. According to this model, atoms have a dense, positively charged nucleus at the center, which contains most of the atom's mass. The electrons, which are negatively charged particles, orbit around the nucleus in specific energy levels.
Therefore, the correct answer is a) The alpha particles used in Rutherford's experiment are positively charged particles.
Which of the following transitions in a hydrogenatom emits the photon of highest frequency? [2000]- a)n = 2 to n = 1
- b)n = 2 to n = 6
- c)n = 6 to n = 2
- d)n = 1 to n = 2
Correct answer is option 'A'. Can you explain this answer?
Which of the following transitions in a hydrogenatom emits the photon of highest frequency? [2000]
a)
n = 2 to n = 1
b)
n = 2 to n = 6
c)
n = 6 to n = 2
d)
n = 1 to n = 2

|
Naveen Menon answered |
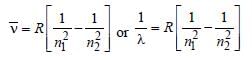
Frequency, 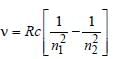
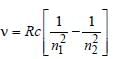
Note : See the greatest energy difference and
also see that the transition is from higher to
lower energy level. Hence, it is highest in
case of n = 2 to n = 1.
also see that the transition is from higher to
lower energy level. Hence, it is highest in
case of n = 2 to n = 1.
Emission line spectra of different elements- a)is not different
- b)is the same if both elements are at the same temperature
- c)is the same if both elements are in liquid form
- d)is different
Correct answer is option 'D'. Can you explain this answer?
Emission line spectra of different elements
a)
is not different
b)
is the same if both elements are at the same temperature
c)
is the same if both elements are in liquid form
d)
is different

|
Adithya Shasan answered |
Option D : Emission line spectra of different elements is different..
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is- a)758 fm
- b)7.58 fm
- c)75.8 fm
- d)0.758 fm
Correct answer is option 'B'. Can you explain this answer?
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is
a)
758 fm
b)
7.58 fm
c)
75.8 fm
d)
0.758 fm
|
|
Riya Banerjee answered |
Correct Answer :- b
Explanation : E = 15.0MeV
= 15 * 106 eV
= 15 * 106 * 1.6 * 10-19 J
= 15 * 1.6 * 10-13 J
E = (1/4πεo)*(ze2/r02)
r0 = (1/4πεo)*(ze2/E)
r0 = (9*109*79*(1.6*10-19)2)/(15*1.6*10-13)
= 75.84 * 10-16 m
= 7.58 fm
In hydrogen atom the angular momentum of the electron in the lowest energy state is- a)2h
- b)h/2π
- c)2π/h
- d)h/π
Correct answer is option 'B'. Can you explain this answer?
In hydrogen atom the angular momentum of the electron in the lowest energy state is
a)
2h
b)
h/2π
c)
2π/h
d)
h/π
|
|
Kiran Khanna answered |
C)h/π
d)h
The correct answer is d) h.
The angular momentum of an electron in the hydrogen atom is given by the formula L = nħ, where n is the principal quantum number and ħ is the reduced Planck constant.
In the lowest energy state, the electron is in the ground state with n = 1. Therefore, the angular momentum is L = 1ħ = h.
d)h
The correct answer is d) h.
The angular momentum of an electron in the hydrogen atom is given by the formula L = nħ, where n is the principal quantum number and ħ is the reduced Planck constant.
In the lowest energy state, the electron is in the ground state with n = 1. Therefore, the angular momentum is L = 1ħ = h.
In Geiger-Marsden experiment very small deflection of the beam was expected because- a)there are no electrical forces at work
- b)positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
- c)particles are collimated by lead screens
- d)most particles pass through
Correct answer is option 'B'. Can you explain this answer?
In Geiger-Marsden experiment very small deflection of the beam was expected because
a)
there are no electrical forces at work
b)
positive charge and the negative electrons are distributed through the whole atom reducing electric field inside the atom
c)
particles are collimated by lead screens
d)
most particles pass through
|
|
Shraddha Choudhury answered |
Explanation:
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
The Geiger-Marsden experiment was conducted to study the structure of an atom. In this experiment, a beam of alpha particles was directed towards a thin gold foil. The alpha particles were expected to pass through the gold foil with little or no deflection, as it was believed that the positive charge and the negative electrons in an atom are distributed uniformly, reducing the electric field inside the atom. However, the results of the experiment were surprising, as some of the alpha particles were deflected at large angles, and some even bounced back.
Reasons for very small deflection of the beam:
- Electrical forces: According to Coulomb's law, any two charged particles exert a force on each other. In an atom, the positively charged nucleus and the negatively charged electrons are attracted to each other by electrical forces. However, the electrons are in constant motion, creating a cloud of negative charge around the nucleus. This cloud of negative charge reduces the electric field inside the atom, making it difficult for the alpha particles to be deflected.
- Distribution of charge: The positive charge in an atom is concentrated in the nucleus, while the negative charge is distributed throughout the atom. This distribution of charge makes the electric field inside the atom more uniform, reducing the chances of the alpha particles being deflected.
- Collimation of particles: The alpha particles were collimated by lead screens before they were directed towards the gold foil. This was done to ensure that the particles were traveling in a straight line and were not scattered by other particles or objects in the environment.
- Most particles pass through: Despite the above factors, it was still expected that some of the alpha particles would be deflected at small angles due to the random nature of the collisions between the particles and the atoms in the gold foil. However, it was not expected that some of the particles would be deflected at large angles or bounce back, as this implied that the positive charge in an atom was not uniformly distributed.
Conclusion:
In conclusion, the very small deflection of the beam was expected in the Geiger-Marsden experiment due to the distribution of charge in an atom and the reduction of electric field inside the atom. However, the unexpected results of the experiment led to the discovery of the nucleus and the development of the modern atomic model.
The ratio of volume of atom to volume of nucleus is- a)1/1000
- b)10
- c)1015
- d)1010
Correct answer is option 'C'. Can you explain this answer?
The ratio of volume of atom to volume of nucleus is
a)
1/1000
b)
10
c)
1015
d)
1010
|
|
Anaya Patel answered |
The ratio of the volume of the atom and the volume of the nucleus is 1015
The radius of an atomic nucleus is of the order of 10−13cm or 10−15m or one Fermi unit.
On the other hand, the radius of an atom is of the order of 10−8cm or 10−10m or one angstrom unit.
Note:
The radius of nucleus is much smaller than atomic radius.
The ratio of atomic radius to radius of nucleus is 10−10m /10−15m =105
Volume is proportional to cube of radius.
The ratio of atomic radius to radius of nucleus is (105)3=1015
The radius of an atomic nucleus is of the order of 10−13cm or 10−15m or one Fermi unit.
On the other hand, the radius of an atom is of the order of 10−8cm or 10−10m or one angstrom unit.
Note:
The radius of nucleus is much smaller than atomic radius.
The ratio of atomic radius to radius of nucleus is 10−10m /10−15m =105
Volume is proportional to cube of radius.
The ratio of atomic radius to radius of nucleus is (105)3=1015
J.J. Thomson’s cathode-ray tube experimentdemonstrated that [2003]- a)the e/m ratio of the cathode-ray particleschanges when a different gas is placed inthe discharge tube
- b)cathode rays are streams of negatively
charged ions - c)all the mass of an atom is essentially in the
nucleus - d)the e/m of electrons is much greater thanthe e/m of protons
Correct answer is option 'B'. Can you explain this answer?
J.J. Thomson’s cathode-ray tube experimentdemonstrated that [2003]
a)
the e/m ratio of the cathode-ray particleschanges when a different gas is placed inthe discharge tube
b)
cathode rays are streams of negatively
charged ions
charged ions
c)
all the mass of an atom is essentially in the
nucleus
nucleus
d)
the e/m of electrons is much greater thanthe e/m of protons

|
Prasenjit Pillai answered |
Cathode rays are streams of negatively
charged ions
charged ions
Absorption line spectrum is obtained- a)If we pass off-white (discrete-spectrum) light through a cool gas
- b)If we pass white (continuous-spectrum) light through a hot gas
- c)If we pass off-white (discrete-spectrum) light through a hot gas
- d)If we pass white (continuous-spectrum) light through a cool gas
Correct answer is option 'D'. Can you explain this answer?
Absorption line spectrum is obtained
a)
If we pass off-white (discrete-spectrum) light through a cool gas
b)
If we pass white (continuous-spectrum) light through a hot gas
c)
If we pass off-white (discrete-spectrum) light through a hot gas
d)
If we pass white (continuous-spectrum) light through a cool gas
|
|
Nishant Sharma answered |
Answer is D
Fluorescence is- a)it consists of accelerated atoms/molecules striking suitable material
- b)it consists only of atoms going into stable excited states
- c)what happens in a fluorescent lamp
- d)it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
Correct answer is option 'D'. Can you explain this answer?
Fluorescence is
a)
it consists of accelerated atoms/molecules striking suitable material
b)
it consists only of atoms going into stable excited states
c)
what happens in a fluorescent lamp
d)
it consists of a molecule, atom or nanostructure relaxing to its ground state by emitting a photon of light after being excited to a higher quantum state by some type of energy
|
|
Nikita Singh answered |
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.
The half life of radium is about 1600 years. Of 100 g of radium existing now, 25 g will remainunchanged after [2004]- a)3200 years
- b)4800 years
- c)6400 years
- d)2400 years
Correct answer is option 'A'. Can you explain this answer?
The half life of radium is about 1600 years. Of 100 g of radium existing now, 25 g will remainunchanged after [2004]
a)
3200 years
b)
4800 years
c)
6400 years
d)
2400 years

|
Maheshwar Saini answered |
100 g will become 25 g in two half lives, so, it
is 3200 years
is 3200 years
The targets used in the alpha particle atomic experiments in the early 1900’s was:- a)metal foils
- b)alpha particles
- c)radioactive sources
- d)gold foil
Correct answer is option 'D'. Can you explain this answer?
The targets used in the alpha particle atomic experiments in the early 1900’s was:
a)
metal foils
b)
alpha particles
c)
radioactive sources
d)
gold foil
|
|
Shreya Singh answered |
It's Rutherford alpha Ray scattering experiment.....in this experiment gold foil was used...
In Bohr model of hydrogen atom, radiation is emitted when the electron- a)jumps from higher orbit into the lower orbit
- b)the electron escapes from the orbit
- c)jumps from lower orbit into the higher orbit
- d)revolves in an orbit
Correct answer is option 'A'. Can you explain this answer?
In Bohr model of hydrogen atom, radiation is emitted when the electron
a)
jumps from higher orbit into the lower orbit
b)
the electron escapes from the orbit
c)
jumps from lower orbit into the higher orbit
d)
revolves in an orbit
|
|
Yamuna Mani answered |
Answer is A
E2-E1=hv
E2-E1=hv
What is the radius of iodine atom (At. no. 53,mass no. 126) [1988]- a)2.5 × 10–11 m
- b)2.5 × 10–9 m
- c)7 × 10–9 m
- d)7 × 10–6 m
Correct answer is option 'A'. Can you explain this answer?
What is the radius of iodine atom (At. no. 53,mass no. 126) [1988]
a)
2.5 × 10–11 m
b)
2.5 × 10–9 m
c)
7 × 10–9 m
d)
7 × 10–6 m

|
Sneha Basak answered |
53 electrons in iodine atom are distributed as
2, 8, 18, 18, 7
∴ n = 5
2, 8, 18, 18, 7
∴ n = 5
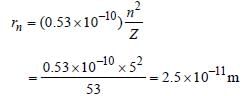
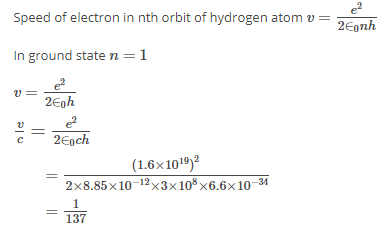
Which of these statements about Bohr model hypothesis is correct?- a)velocity of electron is quantized
- b)mass of electron is quantized
- c)radius of electron is quantized
- d)angular momentum of electron is quantized
Correct answer is option 'D'. Can you explain this answer?
Which of these statements about Bohr model hypothesis is correct?
a)
velocity of electron is quantized
b)
mass of electron is quantized
c)
radius of electron is quantized
d)
angular momentum of electron is quantized
|
|
Krishna Iyer answered |
Bohr never assumed stable electron orbits with the electronic angular momentum quantized as l=mvr=(nh/2π) Quantization of angular momentum means that the radius of the orbit and the energy will be quantized as well. Bohr assumed that the discrete lines seen in the spectrum of the hydrogen atom were due to transitions of an electron from one allowed orbit/energy to another.
In Geiger-Marsden experiment, at the point of closest approach- a)the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
- b)the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
- c)the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
- d)the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
Correct answer is option 'C'. Can you explain this answer?
In Geiger-Marsden experiment, at the point of closest approach
a)
the kinetic energy is not zero and the electrical potential is less than the initial kinetic energy supplied
b)
the kinetic energy is not zero and the electrical potential equals the initial kinetic energy supplied
c)
the kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
d)
the kinetic energy is not zero and the electrical potential is greater than the initial kinetic energy supplied
|
|
Dino James answered |
The kinetic energy is zero and the electrical potential equals the initial kinetic energy supplied
In Rutherford scattering experiment, what willbe the correct angle for α-scattering for an impactparameter, b = 0 ? [1994]- a)90°
- b)270°
- c)0°
- d)180°
Correct answer is option 'D'. Can you explain this answer?
In Rutherford scattering experiment, what willbe the correct angle for α-scattering for an impactparameter, b = 0 ? [1994]
a)
90°
b)
270°
c)
0°
d)
180°

|
Abhijeet Goyal answered |
Impact parameter for Rutherford scattering
experiment,
experiment,
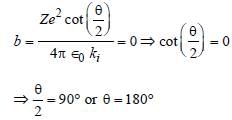
The spectrum obtained from a sodium vapourlamp is an example of [1995]- a)band spectrum
- b)continuous spectrum
- c)emission spectrum
- d)absorption spectrum
Correct answer is option 'C'. Can you explain this answer?
The spectrum obtained from a sodium vapourlamp is an example of [1995]
a)
band spectrum
b)
continuous spectrum
c)
emission spectrum
d)
absorption spectrum

|
Aniket Chawla answered |
A spectrum is observed, when light coming
directly from a source is examined with a
spectroscope. Therefore spectrum obtained
from a sodium vapour lamp is emission
spectrum.
directly from a source is examined with a
spectroscope. Therefore spectrum obtained
from a sodium vapour lamp is emission
spectrum.
The transition from the state n = 3 to n = 1 in ahydrogen like atom results in ultravioletradiation. Infrared radiation will be obtained inthe transition from : [2012M]- a)2 → 1
- b)3 → 2
- c)4 → 2
- d)4 → 3
Correct answer is option 'D'. Can you explain this answer?
The transition from the state n = 3 to n = 1 in ahydrogen like atom results in ultravioletradiation. Infrared radiation will be obtained inthe transition from : [2012M]
a)
2 → 1
b)
3 → 2
c)
4 → 2
d)
4 → 3

|
Deepak Joshi answered |
The frequency of the transition 
when n = 1, 2, 3.
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?- a)7.60 nm
- b)10.60 nm
- c)9.60 nm
- d)8.60 nm
Correct answer is option 'A'. Can you explain this answer?
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?
a)
7.60 nm
b)
10.60 nm
c)
9.60 nm
d)
8.60 nm
|
|
Suresh Iyer answered |
1/ λ =Z2. (both have same transition so same value of n)
λ /122=1/16
λ =122/16
=7.62nm
λ /122=1/16
λ =122/16
=7.62nm
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.- a)656 nm
- b)676 nm
- c)666 nm
- d)686 nm
Correct answer is option 'A'. Can you explain this answer?
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.
a)
656 nm
b)
676 nm
c)
666 nm
d)
686 nm
|
|
Arun Khanna answered |
If that is so grant us some suggestion! Or Balmer sequence (2nd sequence from electrons laying off to n = 2 from bigger than 2) longest wavelength [velocityconstant = frequency x wavelength] is smallest frequency [power = hconstant x frequency] is least enegy is from transition n = 3 to n = 2 or Google it 656.3 nm
Which of these statements about Bohr model applied to hydrogen atom correct?- a)hydrogen atom in its ground level has no magnetic moment due to orbital motion
- b)hydrogen atom in its ground level has magnetic moment due to orbital motion
- c)hydrogen atom in some orbits radiates electromagnetic waves
- d)hydrogen atom in quantized orbits radiates electromagnetic waves
Correct answer is option 'B'. Can you explain this answer?
Which of these statements about Bohr model applied to hydrogen atom correct?
a)
hydrogen atom in its ground level has no magnetic moment due to orbital motion
b)
hydrogen atom in its ground level has magnetic moment due to orbital motion
c)
hydrogen atom in some orbits radiates electromagnetic waves
d)
hydrogen atom in quantized orbits radiates electromagnetic waves
|
|
Swati Verma answered |
A hydrogen atom has magnetic properties because the motion of the electron acts as a current loop. The energy levels of a hydrogen atom associated with orbital angular momentum are split by an external magnetic field because the orbital angular magnetic moment interacts with the field.
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?- a)
15.1 eV - b)13.1 eV
- c)12.1 eV
- d)14.1 eV
Correct answer is option 'C'. Can you explain this answer?
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?
a)
15.1 eV
b)
13.1 eV
c)
12.1 eV
d)
14.1 eV
|
|
Ritu Singh answered |
Energy at ground state E1=−13.6 eV
Energy at n=3: E3=−13.6/9=1.5 eV
To excite it to n=3 energy given to electron is E3−E1=12.1 eV
Energy at n=3: E3=−13.6/9=1.5 eV
To excite it to n=3 energy given to electron is E3−E1=12.1 eV
Which source is associated with a line emissionspectrum ? [1993]- a)Electric fire
- b)Neon street sign
- c)Red traffic light
- d)sun
Correct answer is option 'B'. Can you explain this answer?
Which source is associated with a line emissionspectrum ? [1993]
a)
Electric fire
b)
Neon street sign
c)
Red traffic light
d)
sun

|
Ayush Chavan answered |
Neon street sign is a source of line emission
spectrum.
spectrum.
Average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is- a)about the same as predicted by Rutherford’s model
- b)incomparable to Rutherford’s model
- c)about the more than predicted by Rutherford’s model
- d)about the less than predicted by Rutherford’s model
Correct answer is option 'A'. Can you explain this answer?
Average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is
a)
about the same as predicted by Rutherford’s model
b)
incomparable to Rutherford’s model
c)
about the more than predicted by Rutherford’s model
d)
about the less than predicted by Rutherford’s model
|
|
Priya Patel answered |
About the same
The average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is about the same size as predicted by Rutherford’s model. This is because the average angle was taken in both models.
According to ‘plum pudding model’ atoms on the whole are electrically neutral because- a)positive charge is concentrated at one place and negative charge is elsewhere.
- b)the negative charge of the atom is uniformly distributed throughout the volume of the atom and the positively charged electrons are embedded in it
- c)the positive charge of the atom is uniformly distributed throughout the volume of the atom and the negatively charged electrons are embedded in it
- d)the positive charge of the atom is uniformly distributed throughout the volume of the electron and the negative charge of electrons balances positive parts
Correct answer is option 'C'. Can you explain this answer?
According to ‘plum pudding model’ atoms on the whole are electrically neutral because
a)
positive charge is concentrated at one place and negative charge is elsewhere.
b)
the negative charge of the atom is uniformly distributed throughout the volume of the atom and the positively charged electrons are embedded in it
c)
the positive charge of the atom is uniformly distributed throughout the volume of the atom and the negatively charged electrons are embedded in it
d)
the positive charge of the atom is uniformly distributed throughout the volume of the electron and the negative charge of electrons balances positive parts
|
|
Manoj Chauhan answered |
The plum pudding model is one of several scientific models of the atom. According to J.J. Thomson atomic models the positive particles in the atom form something like the "batter" in a plum pudding, while the negative electrons are scattered through this "batter".
Each element is associated with- a)with a characteristic spectrum of radiation
- b)with a characteristic absorption of monochromatic light
- c)no light radiation or absorption
- d)with a characteristic radiation of monochromatic light
Correct answer is option 'A'. Can you explain this answer?
Each element is associated with
a)
with a characteristic spectrum of radiation
b)
with a characteristic absorption of monochromatic light
c)
no light radiation or absorption
d)
with a characteristic radiation of monochromatic light
|
|
Navya Banerjee answered |
Explanation:
The given question is related to the concept of atomic spectra. Atomic spectra is a characteristic property of every element which is used to identify the element. It is produced when an atom absorbs or emits energy as electromagnetic radiation.
The correct option is A, i.e., each element is associated with a characteristic spectrum of radiation. This means that every element emits or absorbs radiation of a unique wavelength that is specific to that particular element.
The characteristic spectrum of radiation is the set of wavelengths at which an element emits or absorbs radiation. There are three types of atomic spectra:
1. Continuous spectrum: A continuous spectrum is produced when a solid, liquid or dense gas is heated and the atoms in the material emit radiation at all wavelengths. This type of spectrum is not specific to any particular element.
2. Emission spectrum: An emission spectrum is produced when an element is heated or excited with energy, and the electrons in the atom jump to higher energy levels. When these electrons fall back to their original energy levels, they emit energy as radiation. The wavelengths of this radiation are specific to that particular element.
3. Absorption spectrum: An absorption spectrum is produced when an element absorbs certain wavelengths of radiation. When white light is passed through a sample of a specific element, certain wavelengths of light are absorbed by the atoms in the material, leaving dark lines in the spectrum. These dark lines are specific to that particular element.
In summary, the correct option is A because every element is associated with a characteristic spectrum of radiation, which is used to identify the element.
The given question is related to the concept of atomic spectra. Atomic spectra is a characteristic property of every element which is used to identify the element. It is produced when an atom absorbs or emits energy as electromagnetic radiation.
The correct option is A, i.e., each element is associated with a characteristic spectrum of radiation. This means that every element emits or absorbs radiation of a unique wavelength that is specific to that particular element.
The characteristic spectrum of radiation is the set of wavelengths at which an element emits or absorbs radiation. There are three types of atomic spectra:
1. Continuous spectrum: A continuous spectrum is produced when a solid, liquid or dense gas is heated and the atoms in the material emit radiation at all wavelengths. This type of spectrum is not specific to any particular element.
2. Emission spectrum: An emission spectrum is produced when an element is heated or excited with energy, and the electrons in the atom jump to higher energy levels. When these electrons fall back to their original energy levels, they emit energy as radiation. The wavelengths of this radiation are specific to that particular element.
3. Absorption spectrum: An absorption spectrum is produced when an element absorbs certain wavelengths of radiation. When white light is passed through a sample of a specific element, certain wavelengths of light are absorbed by the atoms in the material, leaving dark lines in the spectrum. These dark lines are specific to that particular element.
In summary, the correct option is A because every element is associated with a characteristic spectrum of radiation, which is used to identify the element.
Reason why there are many lines in an atomic spectrum is because- a)All atoms are in the same excited state and make transition to same state
- b)There are many atoms in different states of excitation making transition to the same state
- c)There are many atoms in different states of excitation making transition to different states
- d)All atoms are in the same excited state and make transition to different states
Correct answer is option 'C'. Can you explain this answer?
Reason why there are many lines in an atomic spectrum is because
a)
All atoms are in the same excited state and make transition to same state
b)
There are many atoms in different states of excitation making transition to the same state
c)
There are many atoms in different states of excitation making transition to different states
d)
All atoms are in the same excited state and make transition to different states
|
|
Nishtha Dasgupta answered |
Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states.
The total energy of electron in the ground stateof hydrogen atom is – 13.6 eV. The kinetic energyof an electron in the first excited state is [2007]- a)6.8 eV
- b)13.6 eV
- c)1.7 eV
- d)3.4 eV.
Correct answer is option 'D'. Can you explain this answer?
The total energy of electron in the ground stateof hydrogen atom is – 13.6 eV. The kinetic energyof an electron in the first excited state is [2007]
a)
6.8 eV
b)
13.6 eV
c)
1.7 eV
d)
3.4 eV.

|
Srishti Sen answered |
Energy in the first excited state
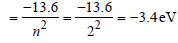
But K.E. = –(Total energy) = +3.4 eV.
When a hydrogen atom is raised from the groundstate to an excited state, [1995]- a)P.E decreases and K.E. increases
- b)P.E. increases and K.E decreases
- c)both K.E. and P.E. decrease
- d)absorption spectrum
Correct answer is option 'B'. Can you explain this answer?
When a hydrogen atom is raised from the groundstate to an excited state, [1995]
a)
P.E decreases and K.E. increases
b)
P.E. increases and K.E decreases
c)
both K.E. and P.E. decrease
d)
absorption spectrum

|
Arnav Iyer answered |
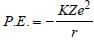 and
and 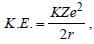 where,
where,r is the radius of orbit which increases as we
move from ground to an excited state.
Therefore, when a hydrogen atom is raised
from the ground state, it increases the value
of r. As a result of this, P.E. increases
(decreases in negative) and K.E. decreases.
If the threshold wavelength for a certain metal is 2000 Å, then the work-function of the metal is [1995]- a)6.2 J
- b)6.2 eV [
- c)6.2 MeV
- d)6.2 keV
Correct answer is option 'B'. Can you explain this answer?
If the threshold wavelength for a certain metal is 2000 Å, then the work-function of the metal is [1995]
a)
6.2 J
b)
6.2 eV [
c)
6.2 MeV
d)
6.2 keV

|
Raghav Khanna answered |
Threshold wavelength (λ) = 2000 Å = 2000 ×
10–10 m. Work function
10–10 m. Work function
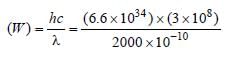

Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?- a)The electrons would spiral inwards and fall into the nucleus
- b)The waves emitted by electrons are discrete
- c)Electrons do not radiate electromagnetic waves
- d)The positive charge and negative electron cancel and no waves are radiated
Correct answer is option 'A'. Can you explain this answer?
Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?
a)
The electrons would spiral inwards and fall into the nucleus
b)
The waves emitted by electrons are discrete
c)
Electrons do not radiate electromagnetic waves
d)
The positive charge and negative electron cancel and no waves are radiated
|
|
Avantika Dasgupta answered |
In classical electromagnetic theory, atoms and molecules are considered to contain electrical charges (i.e. electrons, ions) which are regarded as oscillating about positions of equilibrium, each with its appropriate natural frequency, v0 . When placed in a radiation field of frequency v , each oscillator in the atom or molecule is set into forced vibration with the same frequency as that of the radiation. The amplitude of the forced vibration is small, but as v approaches v0 , the amplitude of the forced vibration increases rapidly. To account for the absorption of energy from the radiation field, it is necessary to assume that the oscillator in the atom or molecule must overcome some frictional force proportional to its velocity during its forced motion. For small amplitudes of forced oscillation, the frictional force, and therefore the absorption of energy, is negligible. Near resonance , the amplitude of oscillation becomes large, with a correspondingly large absorption of energy to overcome the frictional force. Therefore, the radiation of frequencies near the natural frequency of the oscillator corresponds to an absorption band.
Which of these statements about Bohr model hypothesis is correct?- a)angular momentum is not quantized
- b)electron in a stable orbit does not radiate electromagnetic waves
- c)velocity of electron is quantized
- d)electron in a stable orbit emit quanta of light
Correct answer is option 'B'. Can you explain this answer?
Which of these statements about Bohr model hypothesis is correct?
a)
angular momentum is not quantized
b)
electron in a stable orbit does not radiate electromagnetic waves
c)
velocity of electron is quantized
d)
electron in a stable orbit emit quanta of light
|
|
Shraddha Choudhury answered |
Bohr Model Hypothesis and Stable Electron Orbit
Bohr model hypothesis is a model of the atom proposed by Niels Bohr in 1913. It was one of the earliest attempts to explain the structure of atoms and their behavior. The model was based on the assumption that electrons orbit the nucleus in circular paths.
The correct statement about Bohr model hypothesis is:
- Electron in a stable orbit does not radiate electromagnetic waves.
Explanation:
- Electrons in atoms can exist only in certain discrete energy levels. According to Bohr's model, electrons in atoms move around the nucleus in stable orbits, each with a specific energy level.
- Electrons in stable orbits do not emit electromagnetic waves because they are in a stable state and have a fixed amount of energy.
- However, when an electron transitions from a higher energy level to a lower energy level, it emits a photon of light.
- This is because the energy lost by the electron is emitted as a photon of light. The energy of the photon is equal to the difference in energy between the two energy levels.
- Therefore, the Bohr model hypothesis proposed that the electron does not continuously lose energy as it moves in a circular orbit around the nucleus, but instead only loses energy when it transitions between energy levels.
Conclusion:
Bohr model hypothesis is an important model that helps explain the behavior of atoms. The model is based on the assumption that electrons move around the nucleus in stable orbits and only emit electromagnetic waves when they transition between energy levels. The correct statement about the model is that electrons in a stable orbit do not radiate electromagnetic waves.
Bohr model hypothesis is a model of the atom proposed by Niels Bohr in 1913. It was one of the earliest attempts to explain the structure of atoms and their behavior. The model was based on the assumption that electrons orbit the nucleus in circular paths.
The correct statement about Bohr model hypothesis is:
- Electron in a stable orbit does not radiate electromagnetic waves.
Explanation:
- Electrons in atoms can exist only in certain discrete energy levels. According to Bohr's model, electrons in atoms move around the nucleus in stable orbits, each with a specific energy level.
- Electrons in stable orbits do not emit electromagnetic waves because they are in a stable state and have a fixed amount of energy.
- However, when an electron transitions from a higher energy level to a lower energy level, it emits a photon of light.
- This is because the energy lost by the electron is emitted as a photon of light. The energy of the photon is equal to the difference in energy between the two energy levels.
- Therefore, the Bohr model hypothesis proposed that the electron does not continuously lose energy as it moves in a circular orbit around the nucleus, but instead only loses energy when it transitions between energy levels.
Conclusion:
Bohr model hypothesis is an important model that helps explain the behavior of atoms. The model is based on the assumption that electrons move around the nucleus in stable orbits and only emit electromagnetic waves when they transition between energy levels. The correct statement about the model is that electrons in a stable orbit do not radiate electromagnetic waves.
The model that best explains the results of Geiger-Marsden experiment is- a)Thomson model
- b)Thomson model
- c)Rutherford model
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
The model that best explains the results of Geiger-Marsden experiment is
a)
Thomson model
b)
Thomson model
c)
Rutherford model
d)
None of the above
|
|
Sankar Banerjee answered |
When Rutherford saw the results of the experiment by Geiger and Marsden, he said:
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford used the results of this experiment to develop a new model for the atom. This model proposed a central nucleus with a positive charge. It was this positively charged nucleus that was responsible for the strong backward deflection of the positively charged alpha particles.
The model also proposed that negatively charged electrons surrounded this nucleus. However, as most of the alpha particles passed through the gold foil with no deflection at all, Rutherford realised that most of the atom was empty space. So, his model placed the electrons at some distance from the nucleus.
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Rutherford used the results of this experiment to develop a new model for the atom. This model proposed a central nucleus with a positive charge. It was this positively charged nucleus that was responsible for the strong backward deflection of the positively charged alpha particles.
The model also proposed that negatively charged electrons surrounded this nucleus. However, as most of the alpha particles passed through the gold foil with no deflection at all, Rutherford realised that most of the atom was empty space. So, his model placed the electrons at some distance from the nucleus.
When an electron jumps from the fourth orbit tothe second orbit, one gets the [2000]- a)second line of Lyman series
- b)second line of Paschen series
- c)second line of Balmer series
- d)first line of Pfund series
Correct answer is option 'C'. Can you explain this answer?
When an electron jumps from the fourth orbit tothe second orbit, one gets the [2000]
a)
second line of Lyman series
b)
second line of Paschen series
c)
second line of Balmer series
d)
first line of Pfund series

|
Lekshmi Banerjee answered |
When the electron drops from any orbit to
second orbit, then wavelength of line
obtained belongs to Balmer series.
second orbit, then wavelength of line
obtained belongs to Balmer series.
Chapter doubts & questions for Atoms - Topic-wise MCQ Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Atoms - Topic-wise MCQ Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup


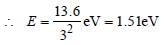

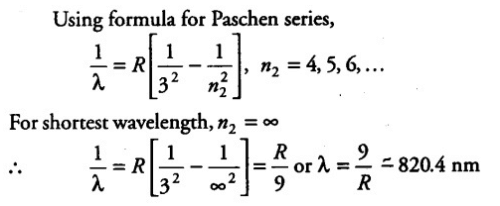

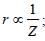 Z(=3) is maximum for Li2+.
Z(=3) is maximum for Li2+.