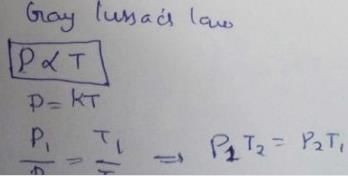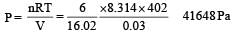All Exams >
NEET >
Physical Chemistry for NEET >
All Questions
All questions of State of Matter for NEET Exam
Graph for Boyle's law is called- a)isotherm
- b)hypertherm
- c)hypotherm
- d)none
Correct answer is option 'A'. Can you explain this answer?
Graph for Boyle's law is called
a)
isotherm
b)
hypertherm
c)
hypotherm
d)
none
|
|
Rohan Singh answered |
Boyle's law: Graph between P and V at constant temperature is called isotherm and is an equilateral (or rectangular) hyperbola. By plotting P versus 1/V, this hyperbola can be converted to a straight line.
A vessel contains 0.5 mole each of SO2, H2 and CH4. Its outlet was made open and closed after some time. Thus, order of partial pressure inside the vessel will be- a)
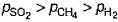
- b)
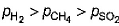
- c)
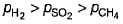
- d)
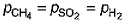
Correct answer is option 'A'. Can you explain this answer?
A vessel contains 0.5 mole each of SO2, H2 and CH4. Its outlet was made open and closed after some time. Thus, order of partial pressure inside the vessel will be
a)
b)
c)
d)

|
Infinity Academy answered |
From Graham's law of diffusion, the rate of diffusion of a gas through a slit or any outlet or inlet in a container, is inversely proportional to the molecular weight of the gas.
So , for a definite time (say t), SO2 will diffuse in the least amount through the outlet as it has highest mass among the three. Similarly the next in the increasing order will be CH4 and finally the last one is H2 will diffuse in the most amount.
Thus, the final amount of gases in the container are in the order SO2 > CH4 > H2.
It's clear that more the amount of the gas, more is its partial pressure.
So, the correct order is P(SO2) > P(CH4) > P(H2)
For Charles’ law, graphical representation between log V and log T under isobaric condition is shown below for equation V = kT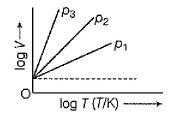 Q. Volume V = constant k, when
Q. Volume V = constant k, when- a)T = 0oc
- b)T = 273oc
- c)T = 0K
- d)T = -272oc
Correct answer is option 'D'. Can you explain this answer?
For Charles’ law, graphical representation between log V and log T under isobaric condition is shown below for equation V = kT
Q. Volume V = constant k, when
a)
T = 0oc
b)
T = 273oc
c)
T = 0K
d)
T = -272oc

|
Ciel Knowledge answered |
-272deg.
For C = 1 K
V / 1 = K
V = K
For C = 1 K
V / 1 = K
V = K
Use of hot air balloons is an application of:- a)Gay Lussac’s law
- b)Avogadro’s law
- c)Charles’ law
- d)Boyle’s law
Correct answer is option 'C'. Can you explain this answer?
Use of hot air balloons is an application of:
a)
Gay Lussac’s law
b)
Avogadro’s law
c)
Charles’ law
d)
Boyle’s law
|
|
Riya Banerjee answered |
The relationship between the temperature and volume of a gas, which is known as Charles' law, provides an explanation of how hot-air balloons work.
Consider the reaction, 2X(g) + 3Y(g) → Z(g)Where gases X and Y are insoluble and inert to water and Z form a basic solution. In an experiment 3 mole each of X and Y are allowed to react in 15 lit flask at 500 K. When the reaction is complete, 5L of water is added to the flask and temperature is reduced to 300 K. The pressure in the flask is (neglect aqueous tension)- a)1.64 atm
- b)2.46 atm
- c) 4.92 atm
- d) 3.28 atm
Correct answer is option 'B'. Can you explain this answer?
Consider the reaction, 2X(g) + 3Y(g) → Z(g)
Where gases X and Y are insoluble and inert to water and Z form a basic solution. In an experiment 3 mole each of X and Y are allowed to react in 15 lit flask at 500 K. When the reaction is complete, 5L of water is added to the flask and temperature is reduced to 300 K. The pressure in the flask is (neglect aqueous tension)
a)
1.64 atm
b)
2.46 atm
c)
4.92 atm
d)
3.28 atm
|
|
Mira Joshi answered |
The reaction is
2X(g) + 3Y(g) → Z(g)
2 moles of X reacts with 3 moles of Y to form 1mole of Z.
Now 3 moles of X and 3 moles of Y are present in the flask. Y is the limiting reagent. (Tocheck LR, divide the no of moles by stoichiometric coff. of that element. The least value will be LR)
After reaction, 1 mole of X is remaining and 1 mole of Z is formed, X is insoluble in water whereas Z is soluble in water.
Thus, the pressure in the flask is due to 1 mole of X.
The total volume of the flask is 15 L. The volume of water is 5 L. Thus the volume of gas is 15-5 = 10L.
The ideal gas equation is pV = nRT
Hence ,
p = nRT/V = 1×0.0821×300/10
= 2.46 atm
2X(g) + 3Y(g) → Z(g)
2 moles of X reacts with 3 moles of Y to form 1mole of Z.
Now 3 moles of X and 3 moles of Y are present in the flask. Y is the limiting reagent. (Tocheck LR, divide the no of moles by stoichiometric coff. of that element. The least value will be LR)
After reaction, 1 mole of X is remaining and 1 mole of Z is formed, X is insoluble in water whereas Z is soluble in water.
Thus, the pressure in the flask is due to 1 mole of X.
The total volume of the flask is 15 L. The volume of water is 5 L. Thus the volume of gas is 15-5 = 10L.
The ideal gas equation is pV = nRT
Hence ,
p = nRT/V = 1×0.0821×300/10
= 2.46 atm
Consider the following pairs of gases A and B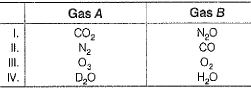 Q. Thus, relative rates of diffusion of gases A and B is in the order
Q. Thus, relative rates of diffusion of gases A and B is in the order - a)Ill < IV < l = II
- b)I = II < Ill < IV
- c)l = Ill < II = IV
- d)I < II < III < IV
Correct answer is option 'A'. Can you explain this answer?
Consider the following pairs of gases A and B
Q. Thus, relative rates of diffusion of gases A and B is in the order
a)
Ill < IV < l = II
b)
I = II < Ill < IV
c)
l = Ill < II = IV
d)
I < II < III < IV
|
|
Anaya Patel answered |
r1 = √(44/44 = 1
r2 = √(44/44) = 1
r3 = √(32/48) = 0.81
r4 = √18/20 = 0.94
So the order is
III < IV < I = II
r2 = √(44/44) = 1
r3 = √(32/48) = 0.81
r4 = √18/20 = 0.94
So the order is
III < IV < I = II
A capillary tube of uniform diameter contains gas samples A and B, separated by a short column of Hg, L mm in length. The ends are sealed. In horizontal position, the confined gases occupy 'a' mm and 'b' mm in length with a common unknown pressure (P). In vertical position, the lengths become respectively a' mm and b' mm. Then P is equal to- a)
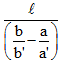
- b)
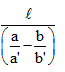
- c)
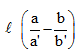
- d)
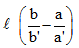
Correct answer is option 'A'. Can you explain this answer?
A capillary tube of uniform diameter contains gas samples A and B, separated by a short column of Hg, L mm in length. The ends are sealed. In horizontal position, the confined gases occupy 'a' mm and 'b' mm in length with a common unknown pressure (P). In vertical position, the lengths become respectively a' mm and b' mm. Then P is equal to
a)
b)
c)
d)
|
|
Krishna Iyer answered |
By ideal gas law: PV=nRT
For a particular gas since n and T are constant. ⇒PV=Constant
⇒ For gas A
Pa=PAa′..............(i) (Here length (a) is directly proportional to volume. Hence it can be used in place of volume.)
⇒ For gas B
Pb=PBb′.......(ii)
Since gas B is supporting l length of mercury over it in addition to gas A
⇒PB=PA+ρgl..........(iii) ( ρ is the density of mercury)
Putting (iii) in (ii)
⇒Pb=(PA+ρgl)b′............(iv)
Eliminating PA
from (i) and (iv)
⇒Pb=Pa/a′b′+ρglb′⇒Pba′−ab′)/a’=ρglb′
⇒P=ρgla′b′/ba’-ab’(in SI units)
⇒P in mm of Hg =dP(in SI)/ρg
P(mmHg)=la′b′/ba′-ab′ = l/(b/b′) - (a/a′)
Thus (a) is the correct option
For a particular gas since n and T are constant. ⇒PV=Constant
⇒ For gas A
Pa=PAa′..............(i) (Here length (a) is directly proportional to volume. Hence it can be used in place of volume.)
⇒ For gas B
Pb=PBb′.......(ii)
Since gas B is supporting l length of mercury over it in addition to gas A
⇒PB=PA+ρgl..........(iii) ( ρ is the density of mercury)
Putting (iii) in (ii)
⇒Pb=(PA+ρgl)b′............(iv)
Eliminating PA
from (i) and (iv)
⇒Pb=Pa/a′b′+ρglb′⇒Pba′−ab′)/a’=ρglb′
⇒P=ρgla′b′/ba’-ab’(in SI units)
⇒P in mm of Hg =dP(in SI)/ρg
P(mmHg)=la′b′/ba′-ab′ = l/(b/b′) - (a/a′)
Thus (a) is the correct option
The vapour pressure of water at 300 K in a closed container is 0.4 atm. If the volume of the container is doubled, its vapour pressure at 300 K will be- a)0.8 atm
- b)0.2 atm
- c)0.4 atm
- d)0.6 atm
Correct answer is option 'C'. Can you explain this answer?
The vapour pressure of water at 300 K in a closed container is 0.4 atm. If the volume of the container is doubled, its vapour pressure at 300 K will be
a)
0.8 atm
b)
0.2 atm
c)
0.4 atm
d)
0.6 atm
|
|
Rohan Singh answered |
The vapour pressure of water will remain same as the temperature is unchanged. So, the answer is c) 0.4 atm
The slope of plot between pV and p at constant temperature is:- a)1
- b)2
- c)1/2
- d)Zero
Correct answer is 'D'. Can you explain this answer?
The slope of plot between pV and p at constant temperature is:
a)
1
b)
2
c)
1/2
d)
Zero
|
|
Shreya Gupta answered |
Slope of the plot between P and PV at constant temperature is zero. A plot of P v/s PV at constant temperature for a fixed mass of gas is a straight line parallel to the pressure axis.

Compressibility factor for CO2 at 400 K and 71.0 bar is 0.8697. Molar volume of CO2 under these conditions is- a)22.4 L
- b)2.24 L
- c)0.41 L
- d)19.5 L
Correct answer is option 'C'. Can you explain this answer?
Compressibility factor for CO2 at 400 K and 71.0 bar is 0.8697. Molar volume of CO2 under these conditions is
a)
22.4 L
b)
2.24 L
c)
0.41 L
d)
19.5 L

|
Lohit Matani answered |
We know that
Z = pVobserved /RT
0.8697 = 71×0.987×Vobserved / 0.0821×400
Vobserved = 0.8697×0.0821×400/71×0.987
= 0.406 L or 0.41 L
Z = pVobserved /RT
0.8697 = 71×0.987×Vobserved / 0.0821×400
Vobserved = 0.8697×0.0821×400/71×0.987
= 0.406 L or 0.41 L
Volume of an ideal gas is to be decreased by 10% by increase of pressure by x% under isothermal condition. Thus, x is- a)100/9
- b)9/100
- c)10
- d)1/10
Correct answer is option 'A'. Can you explain this answer?
Volume of an ideal gas is to be decreased by 10% by increase of pressure by x% under isothermal condition. Thus, x is
a)
100/9
b)
9/100
c)
10
d)
1/10
|
|
Neha Sharma answered |
Applying Boyle 's law P1V1=P2V2 as temp is constant.
So let initial pressure =P initial volume =V
Now final volume V2=V-10%of V =9V/10
PV=P2×9V/10 so P2=10P/9
Therefore increment in Pressure=P/9P×100%=100/9
So let initial pressure =P initial volume =V
Now final volume V2=V-10%of V =9V/10
PV=P2×9V/10 so P2=10P/9
Therefore increment in Pressure=P/9P×100%=100/9
Direction (Q. Nos. 16-19) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).Passage IVolume occupied by molecules is negligible compared to total volume of molecules. We have N2 gas molecules assuming them spherical of radius 200 pm. Q. Total volume of molecules contained in one mole of the gas is- a)22400 cm3
- b)3.721 x 10-22 cm3
- c)4 .65 x 10-23 cm3
- d)20.1 8cm3
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 16-19) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage I
Volume occupied by molecules is negligible compared to total volume of molecules. We have N2 gas molecules assuming them spherical of radius 200 pm.
Q. Total volume of molecules contained in one mole of the gas is
a)
22400 cm3
b)
3.721 x 10-22 cm3
c)
4 .65 x 10-23 cm3
d)
20.1 8cm3
|
|
Riya Banerjee answered |
Volume of single molecule = 4/3π(r)3
= 4/3×π×(200×10-12)3 m3
V = 3.349×10-29 m3
Volume of 1 mole (NA molecules) = 3.349×10-29 m3×6.022×1023
= 2.016×10-5 m3
= 2.016×10-5×106 cm3 or 20.16 cm3
= 4/3×π×(200×10-12)3 m3
V = 3.349×10-29 m3
Volume of 1 mole (NA molecules) = 3.349×10-29 m3×6.022×1023
= 2.016×10-5 m3
= 2.016×10-5×106 cm3 or 20.16 cm3
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which pair of molecules has the strongest dipole-dipole interactions?- a)NH3 and CH4
- b)CH4 and CH4
- c)CO3 and CO2
- d)NH3 and NH3
Correct answer is 'D'. Can you explain this answer?
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which pair of molecules has the strongest dipole-dipole interactions?
a)
NH3 and CH4
b)
CH4 and CH4
c)
CO3 and CO2
d)
NH3 and NH3
|
|
Naina Bansal answered |
NH3 and NH3. Both polar, asymmetric molecules. CH4 is nonpolar. CO2 is symetrical.
A liquid enclosed in 1.0L flask at 298K. The vapour pressure exerted by the liquid is 20.0 mm. when the same liquid is enclosed in a flask of 5L capacity at the same temperature, the vapour pressure of the liquid will be:- a)40mm
- b)20.0mm
- c)10mm
- d)1000mm
Correct answer is option 'B'. Can you explain this answer?
A liquid enclosed in 1.0L flask at 298K. The vapour pressure exerted by the liquid is 20.0 mm. when the same liquid is enclosed in a flask of 5L capacity at the same temperature, the vapour pressure of the liquid will be:
a)
40mm
b)
20.0mm
c)
10mm
d)
1000mm
|
|
Gaurav Kumar answered |
The vapour pressure of a liquid depends only on the nature of the liquid and the temperature. It does not depend on the volume of the container so it is 20.0mm.
At constant temperature, the pressure of a fixed amount of gas varies inversely with its volume, is- a)Boyle’s law
- b)Gay Lussac’s law
- c)Avogadro law
- d)Charles’ law
Correct answer is option 'A'. Can you explain this answer?
At constant temperature, the pressure of a fixed amount of gas varies inversely with its volume, is
a)
Boyle’s law
b)
Gay Lussac’s law
c)
Avogadro law
d)
Charles’ law
|
|
Shreya Gupta answered |
Boyle’s law states that the volume of a fixed mass of a gas is inversely proportional to its pressure at constant temperature.
Mass of gas is 300 gm and its specific heat at constant volume is 750J/kg K. if gas is heated through 75°C at constant pressure of 105 N/m2, it expands by volume 0.08 × 106 cm3. find CP/CV.- a)1.4
- b) 1.374
- c) 1.474
- d)1.5
Correct answer is option 'D'. Can you explain this answer?
Mass of gas is 300 gm and its specific heat at constant volume is 750J/kg K. if gas is heated through 75°C at constant pressure of 105 N/m2, it expands by volume 0.08 × 106 cm3. find CP/CV.
a)
1.4
b)
1.374
c)
1.474
d)
1.5
|
|
Krishna Iyer answered |
Data given;
ΔT = 75 oC = 75 K
ΔV = 0.08 * 106 cm3 = 0.08 m3
Cv = 750 J kg-1 K-1
m = 300 g = 0.300 kg
p = 105 N m-2 = 100000 N m-2
The first law of thermodynamics;
ΔU = Q – W
Q = ΔU + W ---- (1)
mCpΔT = mCvΔT + pΔV ----- (2)
Dividing the above expression (2) with Cv , we get;
Cp / Cv = Cv / Cv + pΔV / mΔTCv
Cp / Cv = 1 + pΔV / mΔTCv
Cp / Cv = 1 + ([100000 N m-2 . 0.08 m3] / [0.300 kg . 75 K .750 J kg-1K-1])
Cp / Cv = 1 + 0.474
Cp / Cv = 1.474 ; After rounding it off we get,
= 1.5 (appx)
ΔT = 75 oC = 75 K
ΔV = 0.08 * 106 cm3 = 0.08 m3
Cv = 750 J kg-1 K-1
m = 300 g = 0.300 kg
p = 105 N m-2 = 100000 N m-2
The first law of thermodynamics;
ΔU = Q – W
Q = ΔU + W ---- (1)
mCpΔT = mCvΔT + pΔV ----- (2)
Dividing the above expression (2) with Cv , we get;
Cp / Cv = Cv / Cv + pΔV / mΔTCv
Cp / Cv = 1 + pΔV / mΔTCv
Cp / Cv = 1 + ([100000 N m-2 . 0.08 m3] / [0.300 kg . 75 K .750 J kg-1K-1])
Cp / Cv = 1 + 0.474
Cp / Cv = 1.474 ; After rounding it off we get,
= 1.5 (appx)
A 8.3 L cylinder contains 128 . 0 g O2 gas at 27°C. What mass of O2 gas must be released to reduce the pressure to 6.0 bar ?- a)64.0 g
- b)32.0 g
- c)16.0 g
- d)8.0 g
Correct answer is option 'A'. Can you explain this answer?
A 8.3 L cylinder contains 128 . 0 g O2 gas at 27°C. What mass of O2 gas must be released to reduce the pressure to 6.0 bar ?
a)
64.0 g
b)
32.0 g
c)
16.0 g
d)
8.0 g
|
|
Rajesh Gupta answered |
By applying ideal gas eqn. PV = nRT
Further solving it we get an eq.
w = PVM/RT
Putting values, we get 64g.
Hence, the correct answer is Option A.
Further solving it we get an eq.
w = PVM/RT
Putting values, we get 64g.
Hence, the correct answer is Option A.
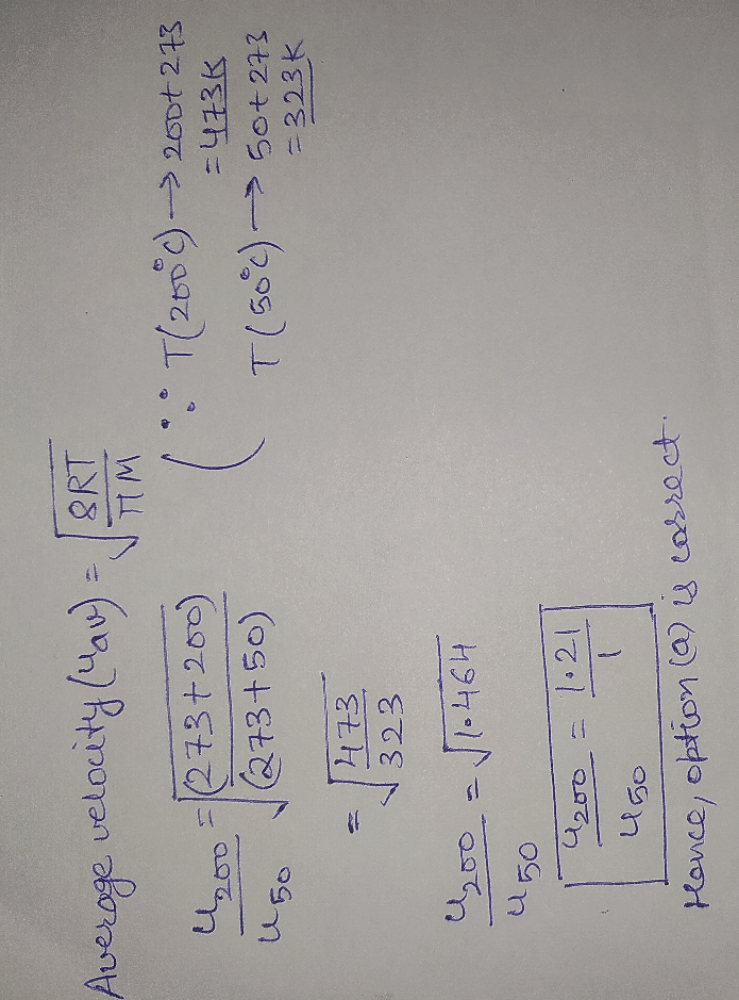
Root mean square velocity (u) is dependent on temperature. Value of 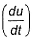 is
is- a)
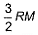
- b)
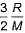
- c)
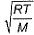
- d)
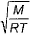
Correct answer is option 'B'. Can you explain this answer?
Root mean square velocity (u) is dependent on temperature. Value of 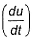 is
is
a)
b)
c)
d)
|
|
Krishna Iyer answered |
The correct answer is option B
Root mean square is v=under root 3RT/M
so we simply do differentiation with respect to temperature DU/DT=D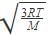 /DT
/DT
So,
= 3R/2M
Root mean square is v=under root 3RT/M
so we simply do differentiation with respect to temperature DU/DT=D
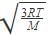 /DT
/DT So,
= 3R/2M
At constant volume, pressure of a fixed amount of a gas varies directly with the temperature, is- a)Gay Lussac’s law
- b)Avogadro law
- c)Boyle’s law
- d)Charles’ law
Correct answer is option 'A'. Can you explain this answer?
At constant volume, pressure of a fixed amount of a gas varies directly with the temperature, is
a)
Gay Lussac’s law
b)
Avogadro law
c)
Boyle’s law
d)
Charles’ law
|
|
Anjali Iyer answered |
Gay Lussac’s law states that the pressure of a fixed mass of a gas at constant volume is directly proportional to the temperature on Kelvin scale.
The value of universal gas constant R depends on:- a)Number of moles of gas
- b)Units of volume and pressure
- c)Volume of gas
- d)Temperature of gas
Correct answer is option 'B'. Can you explain this answer?
The value of universal gas constant R depends on:
a)
Number of moles of gas
b)
Units of volume and pressure
c)
Volume of gas
d)
Temperature of gas
|
|
Pooja Mehta answered |
We have
R = PV/T
= 8.314JK^−1mol^−1
= 8.314x10^7ergK^−1mol^−1
= 2calK^−1mol^−1
= 0.0821litreatmK^−1mol^−1
Hence R depends on units of volume and pressure
Hence answer is (b)
The temperature in Celsius scale can be converted into Kelvin scale- a)By dividing it with 273
- b)By subtracting 273.15 from it
- c)By multiplying it with 273
- d)By adding 273.15 to it
Correct answer is option 'D'. Can you explain this answer?
The temperature in Celsius scale can be converted into Kelvin scale
a)
By dividing it with 273
b)
By subtracting 273.15 from it
c)
By multiplying it with 273
d)
By adding 273.15 to it
|
|
Krishna Iyer answered |
To convert from Celsius to Kelvin you use the following formula:
Celsius temperature + 273.15 = Kelvin Temperature
For example:
26 °Celsius + 273.15 = 299.15 Kelvin
26 °Celsius + 273.15 = 299.15 Kelvin
The Kelvin temperature scale was designed so that it starts at absolute zero. In Kelvin, absolute zero is equal to 0 degrees. In Celsius, absolute zero is equal to −273.15 degrees.
Therefore, you need to add 273.15 to the Celsius temperature to get to the Kelvin temperature.
Note: Kelvin does not use the degree symbol, °.
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of  Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is- a)London interaction
- b)dipole-dipole interaction
- c)hydrogen bonding
- d)dipole-induced dipole interaction
Correct answer is option 'A'. Can you explain this answer?
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
a)
London interaction
b)
dipole-dipole interaction
c)
hydrogen bonding
d)
dipole-induced dipole interaction
|
|
Rajeev Saxena answered |
(a) HF - Hydrogen bonding HCl,HBr,HI→ Dipole-dipole interaction, London-dispersion force.
For real gases van der Waals equation is written as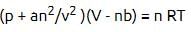 where ‘a’ and ‘b’ are van der Waals constants.
where ‘a’ and ‘b’ are van der Waals constants.
Two sets of gases are :(I) O2, CO2, H2 and He(II) CH4, O2 and H2The gases given in set-I in increasing order of ‘b’ and gases given in set-II in decreasing order of ‘a’, are arranged below. Select the correct order from the following : - a)(I) H2 < He < O2 < CO2 (II) CH4 > O2 > H2
- b)(I) O2 < He < H2 < CO2 (II) H2 > O2 > CH4
- c)(I) He < H2 < CO2 < O2 (II) CH4 > H2 > O2
- d)(I) H2 < O2 < He < CO2 (II) O2 > CH4 > H2
Correct answer is option 'A'. Can you explain this answer?
For real gases van der Waals equation is written as
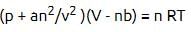
where ‘a’ and ‘b’ are van der Waals constants.
Two sets of gases are :
Two sets of gases are :
(I) O2, CO2, H2 and He
(II) CH4, O2 and H2
The gases given in set-I in increasing order of ‘b’ and gases given in set-II in decreasing order of ‘a’, are arranged below. Select the correct order from the following :
a)
(I) H2 < He < O2 < CO2 (II) CH4 > O2 > H2
b)
(I) O2 < He < H2 < CO2 (II) H2 > O2 > CH4
c)
(I) He < H2 < CO2 < O2 (II) CH4 > H2 > O2
d)
(I) H2 < O2 < He < CO2 (II) O2 > CH4 > H2
|
|
Pooja Shah answered |
Van der Waal gas constant 'a' represent intermolecular force of attraction of gaseous molecules and Van der Waal gas constant 'b' represent effective size of molecules. Therefore order should be (I) H2 < He < O2 < CO2
(II) CH4 > O2 > H2
Density of an ideal gas at 298 K and 1.0 atm is found to be 1.25 kg m-3. Density of the gas at 1.5 atm and at 298 K is - a)1.25 kg m-3
- b)1.875 kg m-3
- c)1.00 kg m-3
- d)1.20 kg m-3
Correct answer is option 'B'. Can you explain this answer?
Density of an ideal gas at 298 K and 1.0 atm is found to be 1.25 kg m-3. Density of the gas at 1.5 atm and at 298 K is
a)
1.25 kg m-3
b)
1.875 kg m-3
c)
1.00 kg m-3
d)
1.20 kg m-3
|
|
Lavanya Menon answered |
We know ideal gas equation,
PV=nRT.
n= m(mass)/M(molecular weight).
PV=m/M RT.
PM=m/v RT.
density (d)=m(mass)/v (volume).
PM=dRT.
d=PM/RT.
here, density is directly proportional to pressure and inversely proportional to temperature...
d1/d2=P1 T2 / P2 T1.
1.25/d2= 1×298/1.5×298.
1.25/d2=1/1.5.
d2= 1.25×1.5.
d2=1.875
PV=nRT.
n= m(mass)/M(molecular weight).
PV=m/M RT.
PM=m/v RT.
density (d)=m(mass)/v (volume).
PM=dRT.
d=PM/RT.
here, density is directly proportional to pressure and inversely proportional to temperature...
d1/d2=P1 T2 / P2 T1.
1.25/d2= 1×298/1.5×298.
1.25/d2=1/1.5.
d2= 1.25×1.5.
d2=1.875
Bond energy of H2 gas is 104 kcal mol-1. The temperature at which average kinetic energy of gaseous H2 molecules is equal to energy required to dissociate the molecules into atoms, is- a)34620 K
- b)34893 K
- c)31200 K
- d)32723 K
Correct answer is option 'B'. Can you explain this answer?
Bond energy of H2 gas is 104 kcal mol-1. The temperature at which average kinetic energy of gaseous H2 molecules is equal to energy required to dissociate the molecules into atoms, is
a)
34620 K
b)
34893 K
c)
31200 K
d)
32723 K
|
|
Preeti Khanna answered |
Bond energy is the energy to break bonds and convert the sample into atoms.
We know that,
K.E of a mol of gas molecule = 3/2 RT
A/Q 3/2 RT = 104 kcal mol-1
3/2×8.314×T = 104×103 x 4.18 J
T = 34893 K
We know that,
K.E of a mol of gas molecule = 3/2 RT
A/Q 3/2 RT = 104 kcal mol-1
3/2×8.314×T = 104×103 x 4.18 J
T = 34893 K
Select the correct statement(s).- a)Cohesive forces are the intermolecular forces between like molecules and adhesive forces are between unlike molecules
- b)A drop maintains its shape if cohesive forces are stronger than adhesive forces
- c)If cohesive forces are weak compared to adhesive forces, drop collapses and spreads into film
- d)Cohesive forces in mercury, consiste of metallic bonds between atoms, are strong; thus it does not wet glass
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Select the correct statement(s).
a)
Cohesive forces are the intermolecular forces between like molecules and adhesive forces are between unlike molecules
b)
A drop maintains its shape if cohesive forces are stronger than adhesive forces
c)
If cohesive forces are weak compared to adhesive forces, drop collapses and spreads into film
d)
Cohesive forces in mercury, consiste of metallic bonds between atoms, are strong; thus it does not wet glass
|
|
Om Desai answered |
a) True, cohesive forces are intermolecular forces between like molecules and adhesive forces between unlike molecules.
b) True, only due to cohesive force, all the molecules of droplets(which are like molecule) are attracted towards each other to form drop
c) True, if the cohesive force becomes weak as to adhesive forces, then there will be no force to bind water molecules and the drop will collapse and spread into film.
d) True, It's only due to cohesive force that mercury doesn’t wet the glass.
b) True, only due to cohesive force, all the molecules of droplets(which are like molecule) are attracted towards each other to form drop
c) True, if the cohesive force becomes weak as to adhesive forces, then there will be no force to bind water molecules and the drop will collapse and spread into film.
d) True, It's only due to cohesive force that mercury doesn’t wet the glass.
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. 50 mL of H2 gas diffuse through a small hole from a vessel in 20 min. Time taken by 40 ml. of O2 gas to diffuse under similar conditions will be- a)12 min
- b)64 min
- c)8 min
- d)32 min
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. 50 mL of H2 gas diffuse through a small hole from a vessel in 20 min. Time taken by 40 ml. of O2 gas to diffuse under similar conditions will be
a)
12 min
b)
64 min
c)
8 min
d)
32 min
|
|
Krishna Iyer answered |
Volume of hydrogen = 50 mL; Time for diffusion (t) = 20 min and volume of oxygen = 40 mL. Rare of diffusion of hydrogen (r1) = 50/20 = 2.5 mL/min Rate of diffusion of oxygen (r2) = 40/t mL/min Since the molecular mass of hydrogen (M1) = 2 and that of oxygen (M2) = 32, therefore :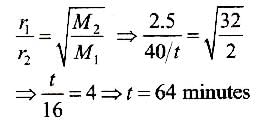
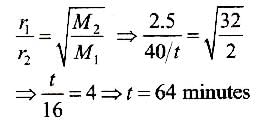
Vapour pressure of mixture of 1 mole volatile liquid A and 1 mole volatile liquid B is 350 mm Hg at 50°C. On adding 2 moles of A Into mixture, vapour pressure increases by 25 mm Hg. Thus, vapour pressure of pure components are (in mm Hg)
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Vapour pressure of mixture of 1 mole volatile liquid A and 1 mole volatile liquid B is 350 mm Hg at 50°C. On adding 2 moles of A Into mixture, vapour pressure increases by 25 mm Hg. Thus, vapour pressure of pure components are (in mm Hg)
a)
a
b)
b
c)
c
d)
d
|
|
Krishna Iyer answered |
We have from Raoult’s law;-
Pmixture = xaPa+xbPb
350 = 1/2×Pa+1/2×Pb
Pa+Pb = 700
And 375 = 3/4Pa+1/4pb
On solving both equations, we get Pa = 400 and Pb = 300
Pmixture = xaPa+xbPb
350 = 1/2×Pa+1/2×Pb
Pa+Pb = 700
And 375 = 3/4Pa+1/4pb
On solving both equations, we get Pa = 400 and Pb = 300
A certain gas effuses out of two different vessels A and B. A has a circular orifice while B has a square orifice of length equal to the radius of the orifice of vessel A. The ratio of rate of diffusion of the gas from vessel A to that from vessel B is - a)π : 1
- b)1 : π
- c)1 : 1
- d)3 : 2
Correct answer is option 'A'. Can you explain this answer?
A certain gas effuses out of two different vessels A and B. A has a circular orifice while B has a square orifice of length equal to the radius of the orifice of vessel A. The ratio of rate of diffusion of the gas from vessel A to that from vessel B is
a)
π : 1
b)
1 : π
c)
1 : 1
d)
3 : 2
|
|
Lavanya Menon answered |
More the area more the effusion.
∴r1/r2 = A1/A2
r1/r2 = πr2/r2 = π
∴r1/r2 = A1/A2
r1/r2 = πr2/r2 = π
A student was given Water, Benzene, Orange juice, and Glycerol to pipette out in four different beakers. The liquid that will be relatively difficult to be sucked into the pipette will be:- a)Orange juice
- b)Water
- c)Benzene
- d)Glycerol
Correct answer is option 'D'. Can you explain this answer?
A student was given Water, Benzene, Orange juice, and Glycerol to pipette out in four different beakers. The liquid that will be relatively difficult to be sucked into the pipette will be:
a)
Orange juice
b)
Water
c)
Benzene
d)
Glycerol
|
|
Krishna Iyer answered |
Glycerol
The reason behind is that it is a viscous liquid.
The reason behind is that it is a viscous liquid.
Rate of diffusion of LPG (mixture of n-butane and propane) is 1.25 times faster than that of SO3. Hence, mole fraction of n-butane in LPG is- a)0.75
- b)0.25
- c)0.51
- d)0.87
Correct answer is option 'C'. Can you explain this answer?
Rate of diffusion of LPG (mixture of n-butane and propane) is 1.25 times faster than that of SO3. Hence, mole fraction of n-butane in LPG is
a)
0.75
b)
0.25
c)
0.51
d)
0.87
|
|
Rahul Bansal answered |
rLPG / rSO3 = √(80/ MLPG) = 1.25
Therefore, 80/M = (1.25)2
Therefore, MLPG = 51.2
M = (M1(butane ) X1 + M2(propane)X2) / (X1 + X2)
51.2 = 58X1 + 44(1-X1) / 1
X1 ≈ 0.51
A cylinder of V litre capacity contains ammonia gas. This cylinder is inverted over another vessel of V litre capacity containing hydrogen chloride at the same temperature and pressure. The pressure in the cylinder after sometime will- a)Remain same
- b)Become 3/2 of the original pressure
- c)Double
- d)Drop
Correct answer is option 'D'. Can you explain this answer?
A cylinder of V litre capacity contains ammonia gas. This cylinder is inverted over another vessel of V litre capacity containing hydrogen chloride at the same temperature and pressure. The pressure in the cylinder after sometime will
a)
Remain same
b)
Become 3/2 of the original pressure
c)
Double
d)
Drop
|
|
Geetika Shah answered |
As per Gay Lussac’s Law, when pressure and temperature are same V directly proportional to the moles. Let n moles of NH3 and n moles HCl after some time they react and form n moles NH4Cl and hence pressure drops.
The standard boiling point of the liquid is
- a)The boiling point at 2 atm pressure.
- b)The boiling point at 1 bar pressure.
- c)The temperature at which a liquid converts to gas.
- d)The boiling point at 1 atm pressure.
Correct answer is option 'B'. Can you explain this answer?
The standard boiling point of the liquid is
a)
The boiling point at 2 atm pressure.
b)
The boiling point at 1 bar pressure.
c)
The temperature at which a liquid converts to gas.
d)
The boiling point at 1 atm pressure.
|
|
Anjana Sharma answered |
The answer is b.
The normal boiling point of liquid is 99.97 degreeC (211.9 degreeF) at a pressure of 1 atm (i.e., 101.325 kPa).
At 298 K, which of the following gases has the lowest average molecular speed?- a)CO2 at 0.20 atm
- b)He at 0.40 atm
- c)CH4 at 0.80 atm
- d)NO at 1.00 atm
Correct answer is option 'A'. Can you explain this answer?
At 298 K, which of the following gases has the lowest average molecular speed?
a)
CO2 at 0.20 atm
b)
He at 0.40 atm
c)
CH4 at 0.80 atm
d)
NO at 1.00 atm
|
|
Preeti Iyer answered |
Under the same conditions the average kinetic energy of the gases should be equal.
speed of gas1/speed of gas 2 = square root of (molar mass of gas2/molar mass of gas 1)
So gases with small molar mass will move quicker
eg speed of N2/speed of CO2 = sq. rt (44/28) = 1.25
speed of N2/speed of F2 = sq rt. (38/28) = 1.16
So order is N2, F2 and CO2
then we concluded right ans. is (A)
speed of gas1/speed of gas 2 = square root of (molar mass of gas2/molar mass of gas 1)
So gases with small molar mass will move quicker
eg speed of N2/speed of CO2 = sq. rt (44/28) = 1.25
speed of N2/speed of F2 = sq rt. (38/28) = 1.16
So order is N2, F2 and CO2
then we concluded right ans. is (A)
A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?- a)3/5
- b)1/ 2
- c)3/ 7
- d)none
Correct answer is option 'A'. Can you explain this answer?
A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?
a)
3/5
b)
1/ 2
c)
3/ 7
d)
none
|
|
Shreya Gupta answered |
Let the volume of air in the flask at 27 degree C be V1 & that of the same amount of the gas at 477 degree C be V2.
According to charle’s law
V2/T2 = V1/ T1 …………………(1)
NOW volume of gas expelled out = V2 – V1, THEN
Fraction of the gas expelled out = (V2 – V1 ) / V2 = 1- (V1/ V2) …………….(2)
Also from equation (1) V1/ V2 = T1/ T2 …………..(3)
Substituting the values of (3) in (2), we get
Fraction of the air expelled = 1- T1/ T2 = (T2 – T1)/ T2
= 750- 300 /750 = 0.6
Hence, fraction of air expelled out is 0.6 or 3/5 th
he number of elements that exists in gaseous state under normal atmospheric conditions is- a)15
- b)10
- c)5
- d)11
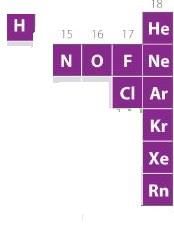
Correct answer is option 'D'. Can you explain this answer?
he number of elements that exists in gaseous state under normal atmospheric conditions is
a)
15
b)
10
c)
5
d)
11
|
|
Hansa Sharma answered |
A look at the periodic table shows us that there are 11 elements in the table that exist in the gaseous state at room temperature. These elements are Hydrogen, Helium, Nitrogen, Oxygen, Fluorine, Chlorine, Neon, Argon, Krypton, Xenon, and Radon.
At what temperature (in °C) root mean square velocity of O2 gas at 300 K is equal to most probable velocity of Ne(20 g mol-1)?
Correct answer is '8'. Can you explain this answer?
At what temperature (in °C) root mean square velocity of O2 gas at 300 K is equal to most probable velocity of Ne(20 g mol-1)?
|
|
Preeti Khanna answered |
Vrms = √(3RT/M)
Vmp 0= √(2RT/M)
Putting corresponding values,
3×300/32 = 2×T/20
On solving, we get T = 281.5K
T in °C = 281.5-273 = 8.1 °C = 8
Vmp 0= √(2RT/M)
Putting corresponding values,
3×300/32 = 2×T/20
On solving, we get T = 281.5K
T in °C = 281.5-273 = 8.1 °C = 8
A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is :- a)0.5 atm
- b)0.8 atm [2011]
- c)0.9 atm
- d)1 atm
Correct answer is option 'A'. Can you explain this answer?
A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is :
a)
0.5 atm
b)
0.8 atm [2011]
c)
0.9 atm
d)
1 atm

|
Abhishek Choudhary answered |
Given nCO = nN2 PCO + PN2 = 1 atm
Partial pressure of a gas = mole fraction of gas × total pressure
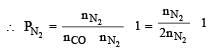

Statement I : In van der Waals’ equation, pressure correction is due to force of attraction between molecules.Statement II : Rate of change of momentum is equal to force.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement i
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement I : In van der Waals’ equation, pressure correction is due to force of attraction between molecules.
Statement II : Rate of change of momentum is equal to force.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement i
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Pioneer Academy answered |
Vander waals constant 'a' is a measure of attractive force
The van der waals constant 'a' represents the magnitude of the attractive forces present between gas molecules. Higher is the value of 'a', more easily the gas can be liquefied.
However it has no relation with statement II. Statement II is a general statement.
The van der waals constant 'a' represents the magnitude of the attractive forces present between gas molecules. Higher is the value of 'a', more easily the gas can be liquefied.
However it has no relation with statement II. Statement II is a general statement.
Mathematical expression that describes Boyle's law is- a)PV = constant
- b)V * constant = P
- c)P * constant = V
- d)V ⁄ P = constant
Correct answer is option 'A'. Can you explain this answer?
Mathematical expression that describes Boyle's law is
a)
PV = constant
b)
V * constant = P
c)
P * constant = V
d)
V ⁄ P = constant

|
Sreeharsha Hareesh answered |
BOYLE'S LAW
At a constant temperature,volume of a definite mass of gas is inversely proportional to its pressure. hence, if P is the pressure and V is the volume, then P x V is a constant.
A 10.0 cm column of air is trapped by a column of Hg 4.00 cm long in a capillary tube of uniform bore when the tube is held horizontally at 1 atm. Length of the air column when the tube is held vertically with the open end up is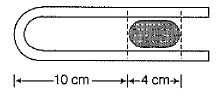
- a)9.50 cm
- b)10.52 cm
- c)3.53cm
- d)4.61 cm
Correct answer is option 'A'. Can you explain this answer?
A 10.0 cm column of air is trapped by a column of Hg 4.00 cm long in a capillary tube of uniform bore when the tube is held horizontally at 1 atm. Length of the air column when the tube is held vertically with the open end up is
a)
9.50 cm
b)
10.52 cm
c)
3.53cm
d)
4.61 cm

|
Lohit Matani answered |
V1 = 10xcm³
V2 = ?
P1 = 1atm = 760mmHg
P2 = (760 + 4)mmHg
P1V1 = P2V2
V2 = P1V1 / P2
= 760 × 10x / 764
= 9.5x
Therefore,
= 9.5x / x = 9.5
V2 = ?
P1 = 1atm = 760mmHg
P2 = (760 + 4)mmHg
P1V1 = P2V2
V2 = P1V1 / P2
= 760 × 10x / 764
= 9.5x
Therefore,
= 9.5x / x = 9.5
Charle's law defines value of 'k' as- a)V/n
- b)V/V
- c)V/P
- d)V/T
Correct answer is option 'D'. Can you explain this answer?
Charle's law defines value of 'k' as
a)
V/n
b)
V/V
c)
V/P
d)
V/T
|
|
Krishna Iyer answered |
According to Charles law,at constant pressure, V∝T
Or V/T = k
Or V/T = k
In comparing gases with liquids , gases have ........ compressibility and...........density.- a)greater, smalle
- b)greater, greater
- c)smaller, smaller
- d)smaller, greater
Correct answer is option 'A'. Can you explain this answer?
In comparing gases with liquids , gases have ........ compressibility and...........density.
a)
greater, smalle
b)
greater, greater
c)
smaller, smaller
d)
smaller, greater
|
|
Neha Patel answered |
In a gas, the distance between molecules, whether monatomic or polyatomic, is very large compared with the size of the molecules; thus gases have a low density and are highly compressible.Density: The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases.
Hydrogen bonding reduces the quality of water molecules to
- a)repel
- b)attract
- c)compactly arrange
- d)slide over each other
Correct answer is option 'D'. Can you explain this answer?
Hydrogen bonding reduces the quality of water molecules to
a)
repel
b)
attract
c)
compactly arrange
d)
slide over each other
|
|
Shreya Gupta answered |
Hydrogen bonding is a type of attractive force that occurs between molecules when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In water molecules, hydrogen bonding occurs between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another water molecule. These hydrogen bonds cause the water molecules to attract each other and stick together, which gives water many of its unique properties, such as its high surface tension and its ability to act as a solvent. The hydrogen bonds do not cause the water molecules to repel each other or to compactly arrange, but they do make it more difficult for the molecules to slide over each other, which contributes to the high viscosity of water.
Chapter doubts & questions for State of Matter - Physical Chemistry for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of State of Matter - Physical Chemistry for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup