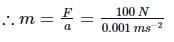All Exams >
NEET >
NCERT Based Tests for NEET >
All Questions
All questions of Units and Measurements for NEET Exam
Which of the following is not a unit of time?- a)Parse
- b)Year
- c)Second
- d)Hour
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a unit of time?
a)
Parse
b)
Year
c)
Second
d)
Hour
|
|
Ayush035 answered |
Distance associated with Parse
The numbers 3.845 and 3.835 on rounding off to 3 significant figures will give- a)3.85 and 3.84
- b)3.84 and 3.83
- c)3.85 and 3.83
- d)3.84 and 3.84
Correct answer is option 'D'. Can you explain this answer?
The numbers 3.845 and 3.835 on rounding off to 3 significant figures will give
a)
3.85 and 3.84
b)
3.84 and 3.83
c)
3.85 and 3.83
d)
3.84 and 3.84
|
|
Mira Joshi answered |
The number 3.845 rounded off to three significant figures becomes 3.84 since the preceding digit is even. On the other hand, the number 3.835 rounded off to three significant figures becomes 3.84 since the preceding digit is odd.
The dimensions of Planck’s constant are the same as that of- a)linear impulse
- b)work
- c)linear momentum
- d)angular momentum
Correct answer is option 'D'. Can you explain this answer?
The dimensions of Planck’s constant are the same as that of
a)
linear impulse
b)
work
c)
linear momentum
d)
angular momentum
|
|
Mira Joshi answered |
Energy of a photon, E = hυ
where h is the Planck's constant and υ is the frequency.

Angular momentum = Moment of inertia x Angular velocity (Angular momentum)
= [ML2][T−1] = [ML2T−1]
where h is the Planck's constant and υ is the frequency.

Angular momentum = Moment of inertia x Angular velocity (Angular momentum)
= [ML2][T−1] = [ML2T−1]
Light year is- a)light emitted by the sun in one year.
- b)the time taken by light to travel from sun to earth.
- c)the distance travelled by light in free space in one year.
- d)the time taken by earth to go once around the sun.
Correct answer is option 'C'. Can you explain this answer?
Light year is
a)
light emitted by the sun in one year.
b)
the time taken by light to travel from sun to earth.
c)
the distance travelled by light in free space in one year.
d)
the time taken by earth to go once around the sun.
|
|
Jyoti Sengupta answered |
Light year is the distance travelled by light in free space in one year.
A body travels uniformly a distance of (13.8 ± 0.2) m in a time (4.0 ± 0.3) s. Its velocity with error limits is- a)(3.5 ± 0.6) m s−1
- b)(3.5 ± 0.3) m s−1
- c)(6.1 ± 0.6) m s−1
- d)(6.1 ± 0.3) m s−1
Correct answer is option 'B'. Can you explain this answer?
A body travels uniformly a distance of (13.8 ± 0.2) m in a time (4.0 ± 0.3) s. Its velocity with error limits is
a)
(3.5 ± 0.6) m s−1
b)
(3.5 ± 0.3) m s−1
c)
(6.1 ± 0.6) m s−1
d)
(6.1 ± 0.3) m s−1
|
|
Dev Patel answered |
Here, s = (13.8 ± 0.2) m, t = (4.0 ± 0.3)s
∴ veliocity, v = s/t = 13.8/4.0 = 3.45 m s−1
(Rounded off to first place of decimal)
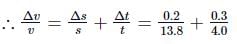
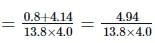
= 0.0865
or Δv = v × 0.0865 = 3.45 × 0.0865
= 0.3087.
∴ velocity =(3.5 ± 0.3) m s−1.
∴ veliocity, v = s/t = 13.8/4.0 = 3.45 m s−1
(Rounded off to first place of decimal)
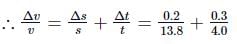
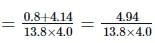
= 0.0865
or Δv = v × 0.0865 = 3.45 × 0.0865
= 0.3087.
∴ velocity =(3.5 ± 0.3) m s−1.
The wrong unit conversion among the following is- a)1 angstrom = 10-10 m
- b)1 fermi = 10-15 m
- c)1 light year = 9.46 x 1015 m
- d)1 astronomical unit = 1.496 x 10-11 m
Correct answer is option 'D'. Can you explain this answer?
The wrong unit conversion among the following is
a)
1 angstrom = 10-10 m
b)
1 fermi = 10-15 m
c)
1 light year = 9.46 x 1015 m
d)
1 astronomical unit = 1.496 x 10-11 m
|
|
Kavya Choudhury answered |
Unit Conversions
Unit conversion is the process of converting one unit of measurement to another. It is important to ensure that the correct unit conversion is used to avoid errors in calculations and measurements.
Given the following unit conversions, the wrong unit conversion is:
a) 1 angstrom = 10-10 m
b) 1 fermi = 10-15 m
c) 1 light year = 9.46 x 1015 m
d) 1 astronomical unit = 1.496 x 10-11 m
Explanation
- Angstrom (Å) to meter (m): 1 Å = 10-10 m. This is a correct conversion.
- Fermi (fm) to meter (m): 1 fm = 10-15 m. This is a correct conversion.
- Light year (ly) to meter (m): 1 ly = 9.46 x 1015 m. This is a correct conversion.
- Astronomical unit (AU) to meter (m): 1 AU = 1.496 x 10-11 m. This is an incorrect conversion. The correct conversion is 1 AU = 1.496 x 108 km.
Conclusion
The wrong unit conversion among the given options is option 'D' which states that 1 astronomical unit is equal to 1.496 x 10-11 m. The correct conversion is 1 astronomical unit is equal to 1.496 x 108 km.
Unit conversion is the process of converting one unit of measurement to another. It is important to ensure that the correct unit conversion is used to avoid errors in calculations and measurements.
Given the following unit conversions, the wrong unit conversion is:
a) 1 angstrom = 10-10 m
b) 1 fermi = 10-15 m
c) 1 light year = 9.46 x 1015 m
d) 1 astronomical unit = 1.496 x 10-11 m
Explanation
- Angstrom (Å) to meter (m): 1 Å = 10-10 m. This is a correct conversion.
- Fermi (fm) to meter (m): 1 fm = 10-15 m. This is a correct conversion.
- Light year (ly) to meter (m): 1 ly = 9.46 x 1015 m. This is a correct conversion.
- Astronomical unit (AU) to meter (m): 1 AU = 1.496 x 10-11 m. This is an incorrect conversion. The correct conversion is 1 AU = 1.496 x 108 km.
Conclusion
The wrong unit conversion among the given options is option 'D' which states that 1 astronomical unit is equal to 1.496 x 10-11 m. The correct conversion is 1 astronomical unit is equal to 1.496 x 108 km.
Which of the following units is not a base unit?- a)metre
- b)candela
- c)ampere
- d)pascal
Correct answer is option 'D'. Can you explain this answer?
Which of the following units is not a base unit?
a)
metre
b)
candela
c)
ampere
d)
pascal
|
|
Dev Patel answered |
Among the given units pascal is the derived unit whereas other are the fundamental or base units.
In which year SI system of units was developed and recommended by General Conference on Weights and Measures?- a)1951
- b)1961
- c)1971
- d)1981
Correct answer is option 'C'. Can you explain this answer?
In which year SI system of units was developed and recommended by General Conference on Weights and Measures?
a)
1951
b)
1961
c)
1971
d)
1981
|
|
Geetika Shah answered |
The SI system of units was developed and recommended by General Conference on Weights and Measures in 1971.
The solid angle subtended by the periphery of an area 1 cm2 at a point situated symmetrically at a distance of 5 cm from the area is- a)2 × 10-2 steradian
- b)4 × 10-2 steradian
- c)6 × 10-2 steradian
- d)8 × 10-2 steradian
Correct answer is option 'B'. Can you explain this answer?
The solid angle subtended by the periphery of an area 1 cm2 at a point situated symmetrically at a distance of 5 cm from the area is
a)
2 × 10-2 steradian
b)
4 × 10-2 steradian
c)
6 × 10-2 steradian
d)
8 × 10-2 steradian
|
|
Anjali Sharma answered |
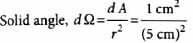
= 4 × 10−2 steradian
If P, Q, R are physical quantities, having different dimensions, which of the following combinations can never be a meaningful quantity?- a)(P - Q)/R
- b)PQ - R
- c)PQ/R
- d)(PR - Q2)/R
Correct answer is option 'A'. Can you explain this answer?
If P, Q, R are physical quantities, having different dimensions, which of the following combinations can never be a meaningful quantity?
a)
(P - Q)/R
b)
PQ - R
c)
PQ/R
d)
(PR - Q2)/R
|
|
Sanaya Mishra answered |
Explanation:
To determine which combination can never be a meaningful quantity, we need to consider the dimensions of the quantities involved.
The dimensions of a physical quantity represent its physical nature and are usually expressed in terms of fundamental dimensions such as length, mass, time, etc.
Let's analyze each combination and determine their dimensions:
a) (P - Q)/R:
The dimensions of (P - Q) will be the same as the dimensions of P and Q since subtraction is only valid between quantities with the same dimensions. Therefore, the dimensions of (P - Q)/R will be the same as the dimensions of P and Q divided by the dimensions of R.
b) PQ - R:
The dimensions of PQ will be the product of the dimensions of P and Q. The dimensions of R will be the same as the dimensions of P and Q since subtraction is only valid between quantities with the same dimensions. Therefore, the dimensions of PQ - R will be the same as the dimensions of P and Q.
c) PQ/R:
The dimensions of PQ will be the product of the dimensions of P and Q. The dimensions of R will be the same as the dimensions of P and Q since division is only valid between quantities with the same dimensions. Therefore, the dimensions of PQ/R will be the same as the dimensions of P and Q divided by the dimensions of R.
d) (PR - Q^2)/R:
The dimensions of PR will be the product of the dimensions of P and R. The dimensions of Q^2 will be the square of the dimensions of Q. The dimensions of R will be the same as the dimensions of P and Q since subtraction and division are only valid between quantities with the same dimensions. Therefore, the dimensions of (PR - Q^2)/R will be the same as the dimensions of P and Q.
From the above analysis, we can conclude that the combination (P - Q)/R can never be a meaningful quantity because subtraction is only valid between quantities with the same dimensions. Therefore, the correct answer is option 'A'.
To determine which combination can never be a meaningful quantity, we need to consider the dimensions of the quantities involved.
The dimensions of a physical quantity represent its physical nature and are usually expressed in terms of fundamental dimensions such as length, mass, time, etc.
Let's analyze each combination and determine their dimensions:
a) (P - Q)/R:
The dimensions of (P - Q) will be the same as the dimensions of P and Q since subtraction is only valid between quantities with the same dimensions. Therefore, the dimensions of (P - Q)/R will be the same as the dimensions of P and Q divided by the dimensions of R.
b) PQ - R:
The dimensions of PQ will be the product of the dimensions of P and Q. The dimensions of R will be the same as the dimensions of P and Q since subtraction is only valid between quantities with the same dimensions. Therefore, the dimensions of PQ - R will be the same as the dimensions of P and Q.
c) PQ/R:
The dimensions of PQ will be the product of the dimensions of P and Q. The dimensions of R will be the same as the dimensions of P and Q since division is only valid between quantities with the same dimensions. Therefore, the dimensions of PQ/R will be the same as the dimensions of P and Q divided by the dimensions of R.
d) (PR - Q^2)/R:
The dimensions of PR will be the product of the dimensions of P and R. The dimensions of Q^2 will be the square of the dimensions of Q. The dimensions of R will be the same as the dimensions of P and Q since subtraction and division are only valid between quantities with the same dimensions. Therefore, the dimensions of (PR - Q^2)/R will be the same as the dimensions of P and Q.
From the above analysis, we can conclude that the combination (P - Q)/R can never be a meaningful quantity because subtraction is only valid between quantities with the same dimensions. Therefore, the correct answer is option 'A'.
Which one of the following physical quantities is not a fundamental quantity?- a)Luminous intensity
- b)Thermodynamic temperature
- c)Electric current
- d)Work
Correct answer is option 'D'. Can you explain this answer?
Which one of the following physical quantities is not a fundamental quantity?
a)
Luminous intensity
b)
Thermodynamic temperature
c)
Electric current
d)
Work
|
|
Jyoti Sengupta answered |
Among the given physical quantities work is not a fundamental quantity whereas all other three physical quantities are fundamental quantities.
The velocity of a particle (v) at an instant t is given by v = at + bt2. The dimension of b is the- a)[L]
- b)[LT-1]
- c)[LT-2]
- d)[LT-3]
Correct answer is option 'D'. Can you explain this answer?
The velocity of a particle (v) at an instant t is given by v = at + bt2. The dimension of b is the
a)
[L]
b)
[LT-1]
c)
[LT-2]
d)
[LT-3]
|
|
Nikhil Sharma answered |
Explanation:
To determine the dimension of the variable "b" in the given equation v = at + bt^2, we need to analyze the dimensions of both sides of the equation.
The dimension of velocity (v) is given by [L][T]^-1, where [L] represents length and [T] represents time.
Dimension of the left-hand side (LHS):
The dimension of velocity (v) is [L][T]^-1.
Dimension of the right-hand side (RHS):
The equation v = at + bt^2 consists of two terms: at and bt^2.
1. Dimension of the first term (at):
The dimension of acceleration (a) is [L][T]^-2.
The dimension of time (t) is [T].
Therefore, the dimension of the first term (at) is [L].
2. Dimension of the second term (bt^2):
The dimension of time (t) is [T].
Therefore, the dimension of the second term (bt^2) is [L][T]^2.
Combining the dimensions:
Since both terms on the RHS have different dimensions, we cannot directly add them. However, for addition to be possible, the dimensions of both terms must be the same.
Comparing the dimensions of the first term (at) and the second term (bt^2), we can see that they have the same dimension of [L].
Therefore, the dimension of b must be such that the dimension of bt^2 is [L]. To cancel out the [T]^2 term, the dimension of b must be [L][T]^-2.
Hence, the correct dimension of b is [LT]^-2, which can be rearranged to [LT]^-3.
Answer:
The correct answer is option D, [LT]^-3.
To determine the dimension of the variable "b" in the given equation v = at + bt^2, we need to analyze the dimensions of both sides of the equation.
The dimension of velocity (v) is given by [L][T]^-1, where [L] represents length and [T] represents time.
Dimension of the left-hand side (LHS):
The dimension of velocity (v) is [L][T]^-1.
Dimension of the right-hand side (RHS):
The equation v = at + bt^2 consists of two terms: at and bt^2.
1. Dimension of the first term (at):
The dimension of acceleration (a) is [L][T]^-2.
The dimension of time (t) is [T].
Therefore, the dimension of the first term (at) is [L].
2. Dimension of the second term (bt^2):
The dimension of time (t) is [T].
Therefore, the dimension of the second term (bt^2) is [L][T]^2.
Combining the dimensions:
Since both terms on the RHS have different dimensions, we cannot directly add them. However, for addition to be possible, the dimensions of both terms must be the same.
Comparing the dimensions of the first term (at) and the second term (bt^2), we can see that they have the same dimension of [L].
Therefore, the dimension of b must be such that the dimension of bt^2 is [L]. To cancel out the [T]^2 term, the dimension of b must be [L][T]^-2.
Hence, the correct dimension of b is [LT]^-2, which can be rearranged to [LT]^-3.
Answer:
The correct answer is option D, [LT]^-3.
Which one of the following statements is incorrect?
- a)Direct and indirect methods are used for the measurement of physical quantities.
- b)Scientific notation and the prefixes are used to simplify numerical computation.
- c)A dimensionally correct equation need not be a correct equation.
- d)The SI units is based on six base units.
Correct answer is option 'D'. Can you explain this answer?
Which one of the following statements is incorrect?
a)
Direct and indirect methods are used for the measurement of physical quantities.
b)
Scientific notation and the prefixes are used to simplify numerical computation.
c)
A dimensionally correct equation need not be a correct equation.
d)
The SI units is based on six base units.
|
|
Dev Patel answered |
Incorrect Statement:
- 4. The SI units are based on six base units.
The SI system actually relies on seven base units:
- Meter (m) for length
- Kilogram (kg) for mass
- Second (s) for time
- Ampere (A) for electric current
- Kelvin (K) for thermodynamic temperature
- Mole (mol) for amount of substance
- Candela (cd) for luminous intensity
Correct Statements:
- 1. Direct and indirect methods are used for the measurement of physical quantities. (True - We can measure directly or use indirect methods like displacement for volume.)
- 2. Scientific notation and the prefixes are used to simplify numerical computation. (True - They help express large/small numbers conveniently.)
- 3. A dimensionally correct equation need not be a correct equation. (True - Matching units doesn't guarantee a factual equation.)
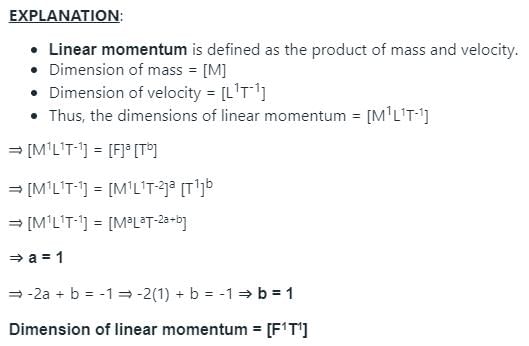
In a certain system, there are only two fundamental quantities - force and time. If their dimensions are [F] and [T] respectively, what are the dimensions of linear momentum in this system?- a)[F1T3]
- b)[F1T1]
- c)[F-1T2]
- d)[F2T2]
Correct answer is option 'B'. Can you explain this answer?
In a certain system, there are only two fundamental quantities - force and time. If their dimensions are [F] and [T] respectively, what are the dimensions of linear momentum in this system?
a)
[F1T3]
b)
[F1T1]
c)
[F-1T2]
d)
[F2T2]
|
|
Rajesh Gupta answered |
Spot out the odd one.- a)calorie
- b)kilowatt hour
- c)joule
- d)watt
Correct answer is option 'D'. Can you explain this answer?
Spot out the odd one.
a)
calorie
b)
kilowatt hour
c)
joule
d)
watt
|
|
Stuti Gupta answered |
Explanation:
Calorie, kilowatt hour, joule:
- Calorie, kilowatt hour, and joule are all units of energy measurement.
- Calorie is a unit of energy commonly used in nutrition to measure the energy content of food.
- Kilowatt hour is a unit of energy used to measure electricity consumption.
- Joule is the SI unit of energy.
Watt:
- Watt, on the other hand, is a unit of power, not energy.
- Power is the rate at which energy is transferred or converted.
- Watt is commonly used to measure the power of electrical devices or the rate at which energy is consumed or produced.
Conclusion:
- While calorie, kilowatt hour, and joule are all units of energy, watt is a unit of power. Therefore, the odd one out in this list is 'watt'.
Calorie, kilowatt hour, joule:
- Calorie, kilowatt hour, and joule are all units of energy measurement.
- Calorie is a unit of energy commonly used in nutrition to measure the energy content of food.
- Kilowatt hour is a unit of energy used to measure electricity consumption.
- Joule is the SI unit of energy.
Watt:
- Watt, on the other hand, is a unit of power, not energy.
- Power is the rate at which energy is transferred or converted.
- Watt is commonly used to measure the power of electrical devices or the rate at which energy is consumed or produced.
Conclusion:
- While calorie, kilowatt hour, and joule are all units of energy, watt is a unit of power. Therefore, the odd one out in this list is 'watt'.
The value of resistance is 10.845 Ω and the current is 3.23 A. On multiplying, we get the potential difference is 35.02935 V. The value of potential difference in terms of significant figures would be- a)35 V
- b)35.0 V
- c)35.029 V
- d)35.03 V
Correct answer is option 'B'. Can you explain this answer?
The value of resistance is 10.845 Ω and the current is 3.23 A. On multiplying, we get the potential difference is 35.02935 V. The value of potential difference in terms of significant figures would be
a)
35 V
b)
35.0 V
c)
35.029 V
d)
35.03 V
|
|
Jyoti Sengupta answered |
The final result should be 3 significant figures.
Which of the following physical quantities has same unit in all the three system of units?- a)Mass
- b)Length
- c)Time
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following physical quantities has same unit in all the three system of units?
a)
Mass
b)
Length
c)
Time
d)
None of these
|
|
Niti Sharma answered |
Time is the physical quantity that has the same unit in all the three systems of units, i.e., CGS (Centimeter-Gram-Second) system, FPS (Foot-Pound-Second) system, and SI (Systeme International) system.
CGS System:
In the CGS system, the unit of time is second (s).
FPS System:
In the FPS system, the unit of time is second (s).
SI System:
In the SI system, the unit of time is second (s).
Explanation:
Mass and length have different units in different systems of units. For example, the unit of mass in CGS system is gram (g), in FPS system it is pound (lb), and in SI system it is kilogram (kg). Similarly, the unit of length in CGS system is centimeter (cm), in FPS system it is foot (ft), and in SI system it is meter (m). Therefore, time is the only physical quantity that has the same unit in all the three systems of units.
CGS System:
In the CGS system, the unit of time is second (s).
FPS System:
In the FPS system, the unit of time is second (s).
SI System:
In the SI system, the unit of time is second (s).
Explanation:
Mass and length have different units in different systems of units. For example, the unit of mass in CGS system is gram (g), in FPS system it is pound (lb), and in SI system it is kilogram (kg). Similarly, the unit of length in CGS system is centimeter (cm), in FPS system it is foot (ft), and in SI system it is meter (m). Therefore, time is the only physical quantity that has the same unit in all the three systems of units.
10-3 gram is called- a)kilogram
- b)milligram
- c)decigram
- d)microgram
Correct answer is option 'B'. Can you explain this answer?
10-3 gram is called
a)
kilogram
b)
milligram
c)
decigram
d)
microgram
|
|
Jyoti Sengupta answered |
1 milligram (mg) = 10-3 gram (g)
The displacement of a progressive wave is represented by y = A sin(ωt - kx) where x is distance and t is time. The dimensions of ω/k are same as those of the- a)velocity
- b)wave number
- c)wavelength
- d)frequency
Correct answer is option 'A'. Can you explain this answer?
The displacement of a progressive wave is represented by y = A sin(ωt - kx) where x is distance and t is time. The dimensions of ω/k are same as those of the
a)
velocity
b)
wave number
c)
wavelength
d)
frequency
|
|
Mohit Choudhury answered |
Explanation:
Dimensions of ω/k:
The dimensions of a quantity are the physical units in which it is measured. In this case, we need to determine the dimensions of the ratio ω/k in the given wave equation y = A sin(ωt - kx).
Relation with Velocity:
The velocity of a wave is given by the ratio of the angular frequency (ω) to the wave number (k) in the form v = ω/k. Therefore, the dimensions of ω/k are the same as the dimensions of velocity.
Conclusion:
Hence, the correct answer is option 'A' - velocity. The dimensions of ω/k are the same as those of velocity.
Dimensions of ω/k:
The dimensions of a quantity are the physical units in which it is measured. In this case, we need to determine the dimensions of the ratio ω/k in the given wave equation y = A sin(ωt - kx).
Relation with Velocity:
The velocity of a wave is given by the ratio of the angular frequency (ω) to the wave number (k) in the form v = ω/k. Therefore, the dimensions of ω/k are the same as the dimensions of velocity.
Conclusion:
Hence, the correct answer is option 'A' - velocity. The dimensions of ω/k are the same as those of velocity.
If power (P), surface tension (S) and Planck’s constant (h) are arranged so that the dimensions of time in their dimensional formulae are in ascending order, then which of the following is correct?- a)P, S, h
- b)P, h, S
- c)S, P, h
- d)S, h, P
Correct answer is option 'A'. Can you explain this answer?
If power (P), surface tension (S) and Planck’s constant (h) are arranged so that the dimensions of time in their dimensional formulae are in ascending order, then which of the following is correct?
a)
P, S, h
b)
P, h, S
c)
S, P, h
d)
S, h, P
|
|
Ananya Das answered |


The ascending order of dimensions of time in their dimensional formulae is P, S, h.
A dimensionless quantity- a)never has a unit
- b)always has unit
- c)may have a unit
- d)does not exit
Correct answer is option 'C'. Can you explain this answer?
A dimensionless quantity
a)
never has a unit
b)
always has unit
c)
may have a unit
d)
does not exit
|
|
Rhea Sharma answered |
Understanding Dimensionless Quantities
Dimensionless quantities are unique in the realm of physics and mathematics, as they do not possess any physical dimensions. Here is an explanation of why option 'C' is the correct answer:
Definition of Dimensionless Quantity
- A dimensionless quantity is a number without any associated unit of measurement.
- It is often used to describe ratios or coefficients that are independent of the system of units.
Examples of Dimensionless Quantities
- Reynolds Number: This is a ratio used in fluid mechanics to predict flow patterns and is calculated using velocity, length, and viscosity but results in a dimensionless number.
- Strain: In mechanics, strain is defined as the ratio of change in length to the original length and is also dimensionless.
Possible Units for Dimensionless Quantities
- Although dimensionless quantities do not have units, they can be expressed in terms of ratios of the same units.
- For example, when calculating a ratio of two lengths (like height to height), the units cancel out, resulting in a dimensionless number.
Conclusion
- Hence, while dimensionless quantities themselves do not carry units, they may be derived from quantities that do have units.
- Therefore, the correct choice is option 'C' – a dimensionless quantity may have a unit, depending on how it is derived or expressed in relation to other quantities.
This understanding is crucial in fields like physics and engineering, where dimensionless numbers play a significant role in analysis and comparisons.
Dimensionless quantities are unique in the realm of physics and mathematics, as they do not possess any physical dimensions. Here is an explanation of why option 'C' is the correct answer:
Definition of Dimensionless Quantity
- A dimensionless quantity is a number without any associated unit of measurement.
- It is often used to describe ratios or coefficients that are independent of the system of units.
Examples of Dimensionless Quantities
- Reynolds Number: This is a ratio used in fluid mechanics to predict flow patterns and is calculated using velocity, length, and viscosity but results in a dimensionless number.
- Strain: In mechanics, strain is defined as the ratio of change in length to the original length and is also dimensionless.
Possible Units for Dimensionless Quantities
- Although dimensionless quantities do not have units, they can be expressed in terms of ratios of the same units.
- For example, when calculating a ratio of two lengths (like height to height), the units cancel out, resulting in a dimensionless number.
Conclusion
- Hence, while dimensionless quantities themselves do not carry units, they may be derived from quantities that do have units.
- Therefore, the correct choice is option 'C' – a dimensionless quantity may have a unit, depending on how it is derived or expressed in relation to other quantities.
This understanding is crucial in fields like physics and engineering, where dimensionless numbers play a significant role in analysis and comparisons.
The order of magnitude of the diameter of the earth is (Diameter of the earth is 1.28 x 107 m)- a)5
- b)6
- c)7
- d)8
Correct answer is option 'C'. Can you explain this answer?
The order of magnitude of the diameter of the earth is (Diameter of the earth is 1.28 x 107 m)
a)
5
b)
6
c)
7
d)
8
|
|
Dev Patel answered |
The order of magnitude of the diameter of the earth is 7.
Which one of the following is not a unit of British system of units?- a)Foot
- b)Metre
- c)Pound
- d)Second
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is not a unit of British system of units?
a)
Foot
b)
Metre
c)
Pound
d)
Second
|
|
Raghav Bansal answered |
Among the given units metre is not a unit of British system whereas all other belong to this system.
The ratio of the volume of the atom to the volume of the nucleus is of the order of- a)1010
- b)1015
- c)1020
- d)1025
Correct answer is option 'B'. Can you explain this answer?
The ratio of the volume of the atom to the volume of the nucleus is of the order of
a)
1010
b)
1015
c)
1020
d)
1025
|
|
Gaurav Kumar answered |
Radius of the atom, Ra = 1 Å = 10-10 m
Volume of the atom,
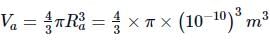
Radius of the nucleus, Rn = 1 fermi = 10−15m
Volume of the atom,
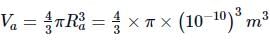
Radius of the nucleus, Rn = 1 fermi = 10−15m
Volume of the nucleus,
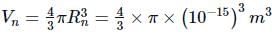
Their corresponding ratio is
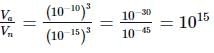
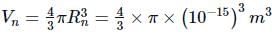
Their corresponding ratio is
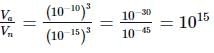
A new system of units is proposed in which unit of mass is α kg, unit of length is β m and unit of time is γ s. What will be value of 5 J in this new system?- a)5αβ2γ−2
- b)5α−1β-2γ2
- c)5α−2β−1γ−2
- d)5α−1 β2γ2
Correct answer is option 'B'. Can you explain this answer?
A new system of units is proposed in which unit of mass is α kg, unit of length is β m and unit of time is γ s. What will be value of 5 J in this new system?
a)
5αβ2γ−2
b)
5α−1β-2γ2
c)
5α−2β−1γ−2
d)
5α−1 β2γ2
|
|
Anjali Sharma answered |
Joule is a unit of energy.
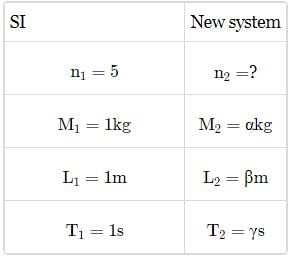
Dimensional formula of energy is [ML2 T−2].
Comparing with [Ma Lb Tc], we get a = 1, b = 2,c = -2
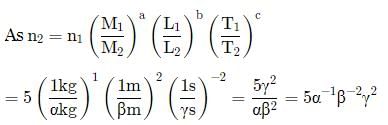
The respective number of significant figures for the numbers 6.320, 6.032, 0.0006032 are- a)3, 4, 8
- b)4, 4, 8
- c)4, 4, 4
- d)4, 3, 4
Correct answer is option 'C'. Can you explain this answer?
The respective number of significant figures for the numbers 6.320, 6.032, 0.0006032 are
a)
3, 4, 8
b)
4, 4, 8
c)
4, 4, 4
d)
4, 3, 4
|
|
Anjali Sharma answered |
According to the rules of significant figures 6.320 has four significant figures.
6.032 has four significant figures. 0.0006032 has four significant figures.
6.032 has four significant figures. 0.0006032 has four significant figures.
The mass of a box measured by a grocer's balance is 2.3 kg. Two gold pieces of masses 20.15 g and 20.17 g are added to the box. The total mass of the box is:- a)2.3 kg
- b)2.34 kg
- c)2.340 kg
- d)2.3403 k
Correct answer is option 'A'. Can you explain this answer?
The mass of a box measured by a grocer's balance is 2.3 kg. Two gold pieces of masses 20.15 g and 20.17 g are added to the box. The total mass of the box is:
a)
2.3 kg
b)
2.34 kg
c)
2.340 kg
d)
2.3403 k
|
|
Dev Patel answered |
Here, mass of the box, m = 2.3 kg
Mass of one gold piece, m1 = 20.15 g = 0.02015 kg
Mass of other gold piece, m2 = 20.17 g = 0.02017 kg
∴ Total mass = m + m1 + m2
= 2.3 kg + 0.02015 kg + 0.02017 kg = 2.34032 kg
As the result is correct only upto one place of decimal, therefore, on rounding off, we get
Total mass = 2.3 kg
Mass of one gold piece, m1 = 20.15 g = 0.02015 kg
Mass of other gold piece, m2 = 20.17 g = 0.02017 kg
∴ Total mass = m + m1 + m2
= 2.3 kg + 0.02015 kg + 0.02017 kg = 2.34032 kg
As the result is correct only upto one place of decimal, therefore, on rounding off, we get
Total mass = 2.3 kg
Which of the following relations is dimensionally incorrect?
- a)1 u = 931.5 MeV/c2
- b)1 u = 931.5 MeV
- c)1 u = 1.67 x 10-27kg
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
Which of the following relations is dimensionally incorrect?
a)
1 u = 931.5 MeV/c2
b)
1 u = 931.5 MeV
c)
1 u = 1.67 x 10-27kg
d)
None of these
|
|
Madhavan Verma answered |
Explanation:
To determine which of the given relations is dimensionally incorrect, we need to check if the units on both sides of the equation are consistent.
Relation a:
1 u = 931.5 MeV/c²
The unit on the left side is atomic mass unit (u), and the unit on the right side is MeV/c² (megaelectronvolt per square of the speed of light). These units are consistent because MeV/c² is commonly used to express the mass-energy equivalence in particle physics. Therefore, relation a is dimensionally correct.
Relation b:
1 u = 931.5 MeV
The unit on the left side is atomic mass unit (u), and the unit on the right side is MeV (megaelectronvolt). These units are not consistent because atomic mass unit represents mass, while MeV represents energy. Therefore, relation b is dimensionally incorrect.
Relation c:
1 u = 1.67 x 10⁻²⁷ kg
The unit on the left side is atomic mass unit (u), and the unit on the right side is kg (kilogram). These units are consistent because atomic mass unit is defined as 1/12th the mass of a carbon-12 atom, and the kilogram is the SI unit of mass. Therefore, relation c is dimensionally correct.
Conclusion:
Among the given relations, relation b (1 u = 931.5 MeV) is dimensionally incorrect because the units on both sides of the equation are not consistent.
To determine which of the given relations is dimensionally incorrect, we need to check if the units on both sides of the equation are consistent.
Relation a:
1 u = 931.5 MeV/c²
The unit on the left side is atomic mass unit (u), and the unit on the right side is MeV/c² (megaelectronvolt per square of the speed of light). These units are consistent because MeV/c² is commonly used to express the mass-energy equivalence in particle physics. Therefore, relation a is dimensionally correct.
Relation b:
1 u = 931.5 MeV
The unit on the left side is atomic mass unit (u), and the unit on the right side is MeV (megaelectronvolt). These units are not consistent because atomic mass unit represents mass, while MeV represents energy. Therefore, relation b is dimensionally incorrect.
Relation c:
1 u = 1.67 x 10⁻²⁷ kg
The unit on the left side is atomic mass unit (u), and the unit on the right side is kg (kilogram). These units are consistent because atomic mass unit is defined as 1/12th the mass of a carbon-12 atom, and the kilogram is the SI unit of mass. Therefore, relation c is dimensionally correct.
Conclusion:
Among the given relations, relation b (1 u = 931.5 MeV) is dimensionally incorrect because the units on both sides of the equation are not consistent.
The relative density of lead is 11.3. Its density in SI unit is - a)1.13 × 103 Kg/m3
- b)1.13 × 102 Kg/m3
- c)1.13 × 104 Kg/m3
- d)11.3 Kg/m3
Correct answer is option 'C'. Can you explain this answer?
The relative density of lead is 11.3. Its density in SI unit is
a)
1.13 × 103 Kg/m3
b)
1.13 × 102 Kg/m3
c)
1.13 × 104 Kg/m3
d)
11.3 Kg/m3
|
|
Anu Mukherjee answered |
Understanding Relative Density
Relative density (also known as specific gravity) is a dimensionless quantity that compares the density of a substance to the density of water at a specific temperature (usually 4°C). For lead, the relative density is given as 11.3.
Calculating Density in SI Units
To find the density of lead in SI units (Kg/m³), we use the following relationship:
- The density of water is approximately 1000 Kg/m³.
- The formula for relative density is:
Relative Density = Density of Substance / Density of Water
By rearranging this formula, we can calculate the density of lead:
- Density of Lead = Relative Density × Density of Water
- Density of Lead = 11.3 × 1000 Kg/m³ = 11,300 Kg/m³
Interpreting the Answer
The calculated density of lead is 11,300 Kg/m³. In scientific notation, this can be expressed as:
- 11,300 Kg/m³ = 1.13 × 10^4 Kg/m³
Thus, the correct answer is option 'C' (1.13 × 10^4 Kg/m³).
Options Overview
- a) 1.13 × 10³ Kg/m³ → Incorrect
- b) 1.13 × 10² Kg/m³ → Incorrect
- c) 1.13 × 10⁴ Kg/m³ → Correct
- d) 11.3 Kg/m³ → Incorrect
Conclusion
Hence, the density of lead in SI units is indeed 1.13 × 10^4 Kg/m³, confirming that option 'C' is the right choice. Understanding these conversions is essential for physics and chemistry, especially in examinations like NEET.
Relative density (also known as specific gravity) is a dimensionless quantity that compares the density of a substance to the density of water at a specific temperature (usually 4°C). For lead, the relative density is given as 11.3.
Calculating Density in SI Units
To find the density of lead in SI units (Kg/m³), we use the following relationship:
- The density of water is approximately 1000 Kg/m³.
- The formula for relative density is:
Relative Density = Density of Substance / Density of Water
By rearranging this formula, we can calculate the density of lead:
- Density of Lead = Relative Density × Density of Water
- Density of Lead = 11.3 × 1000 Kg/m³ = 11,300 Kg/m³
Interpreting the Answer
The calculated density of lead is 11,300 Kg/m³. In scientific notation, this can be expressed as:
- 11,300 Kg/m³ = 1.13 × 10^4 Kg/m³
Thus, the correct answer is option 'C' (1.13 × 10^4 Kg/m³).
Options Overview
- a) 1.13 × 10³ Kg/m³ → Incorrect
- b) 1.13 × 10² Kg/m³ → Incorrect
- c) 1.13 × 10⁴ Kg/m³ → Correct
- d) 11.3 Kg/m³ → Incorrect
Conclusion
Hence, the density of lead in SI units is indeed 1.13 × 10^4 Kg/m³, confirming that option 'C' is the right choice. Understanding these conversions is essential for physics and chemistry, especially in examinations like NEET.
A cube has a side of length 1.2 x 10-2m. Its volume upto correct significant figures is- a)1.7 x 10-6 m3
- b)1.73 x 10-6 m3
- c)1.78 x 10-6 m3
- d)1.732 x 10-6 m3
Correct answer is option 'A'. Can you explain this answer?
A cube has a side of length 1.2 x 10-2m. Its volume upto correct significant figures is
a)
1.7 x 10-6 m3
b)
1.73 x 10-6 m3
c)
1.78 x 10-6 m3
d)
1.732 x 10-6 m3
|
|
Vivek Patel answered |
Here
Length of the cube, L = 1.2 x 10-2 m
Volume of the cube, V = (1.2 x 10-2m)3 = 1.728 x 10-6 m3
As the result can have only two significant figures, therefore, on rounding off, we get, V = 1.7 x 10-6 m3
Length of the cube, L = 1.2 x 10-2 m
Volume of the cube, V = (1.2 x 10-2m)3 = 1.728 x 10-6 m3
As the result can have only two significant figures, therefore, on rounding off, we get, V = 1.7 x 10-6 m3
Which of the following statements is incorrect regarding significant figures?- a)All the non-zero digits are significant
- b)All the zeros between two non-zero digits are significant
- c)Greater the number of significant figures in a measurement smaller is the percentage error
- d)The power of 10 is counted while counting the number of significant figures
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements is incorrect regarding significant figures?
a)
All the non-zero digits are significant
b)
All the zeros between two non-zero digits are significant
c)
Greater the number of significant figures in a measurement smaller is the percentage error
d)
The power of 10 is counted while counting the number of significant figures
|
|
Ajay Yadav answered |
The power of 10 is irrelevant to the determination of significant figures.
Which of the following physical quantities has a unit but no dimensions?- a)Relative velocity
- b)Relative density
- c)Strain
- d)Angle
Correct answer is option 'D'. Can you explain this answer?
Which of the following physical quantities has a unit but no dimensions?
a)
Relative velocity
b)
Relative density
c)
Strain
d)
Angle
|
|
Mira Joshi answered |
Among the given physical quantities angle has a unit but no dimensions. Angle = [M°L°T°]
The SI unit of angle is radian.
The SI unit of angle is radian.
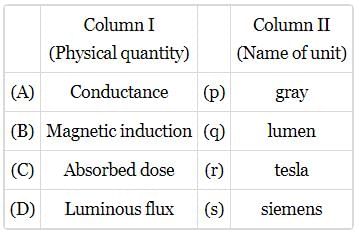
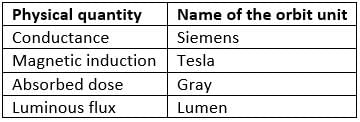
Match the Column I with Column II.

- a)A−q, B−p, C−r, D−s
- b)A−p, B−q, C−s, D−r
- c)A−r, B−s, C−p, D−q
- d)A−s, B−r, C−q, D−p
Correct answer is option 'C'. Can you explain this answer?
Match the Column I with Column II.


a)
A−q, B−p, C−r, D−s
b)
A−p, B−q, C−s, D−r
c)
A−r, B−s, C−p, D−q
d)
A−s, B−r, C−q, D−p
|
|
Dev Patel answered |
(A) Pa s = [ML−1 T−2][T] = [ML−1T−1]
A - r

A - r

The vernier scale of a travelling microscope has 50 divisions which coincide with 49 main scale divisions. If each main scale division is 0.5 mm, then the least count of the microscope is- a)0.02 mm
- b)0.05 mm
- c)0.01 mm
- d)0.1 mm
Correct answer is option 'C'. Can you explain this answer?
The vernier scale of a travelling microscope has 50 divisions which coincide with 49 main scale divisions. If each main scale division is 0.5 mm, then the least count of the microscope is
a)
0.02 mm
b)
0.05 mm
c)
0.01 mm
d)
0.1 mm
|
|
Jyoti Sengupta answered |
1 MSD = 0.5mm
50VSD = 49MSD
1 VSD = 49/50 MSD
Least Count = 1 MSD - 1 VSD
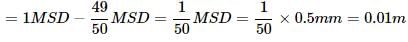
50VSD = 49MSD
1 VSD = 49/50 MSD
Least Count = 1 MSD - 1 VSD
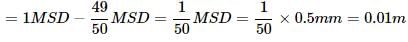
Which of the following is unitless quantity?- a)Pressure gradient
- b)Displacement gradient
- c)Force gardient
- d)Velocity gradient
Correct answer is option 'B'. Can you explain this answer?
Which of the following is unitless quantity?
a)
Pressure gradient
b)
Displacement gradient
c)
Force gardient
d)
Velocity gradient
|
|
Hansa Sharma answered |
Among the given quantities displacement gradient is a unitless quantity.
The ratio of molar volume to atomic volume for 1 mole of hydrogen is (Take size of hydrogen molecule to be 1 Å)- a)7.1 x 104
- b)7.1 x 106
- c)7.1 x 1010
- d)7.1 x 108
Correct answer is option 'A'. Can you explain this answer?
The ratio of molar volume to atomic volume for 1 mole of hydrogen is (Take size of hydrogen molecule to be 1 Å)
a)
7.1 x 104
b)
7.1 x 106
c)
7.1 x 1010
d)
7.1 x 108
|
|
Riya Banerjee answered |
Volume occupied by 1 mole of an ideal gas at STP is known as molar volume.
∴ Molar volume = 22.4 litre = 22.4 x 10-3 m3
Radius of hydrogen atom is r = 0.5 Å = 0.5 x 10-10 m

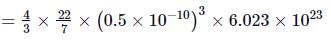
= 3.15 x 10-7 m3
Their corresponding ratio is
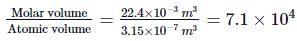
∴ Molar volume = 22.4 litre = 22.4 x 10-3 m3
Radius of hydrogen atom is r = 0.5 Å = 0.5 x 10-10 m

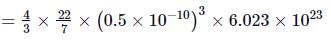
= 3.15 x 10-7 m3
Their corresponding ratio is
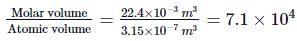
Which of the following statements is incorrect?- a)Every measurement by measuring instrument has some error.
- b)A measurement can have more accuracy but less precision and vice versa.
- c)Every calculated quantity that is based on measured values has some error.
- d)The magnitude of the difference between the true value of the quantity and the individual measurement value is called the relative error of the measurement.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements is incorrect?
a)
Every measurement by measuring instrument has some error.
b)
A measurement can have more accuracy but less precision and vice versa.
c)
Every calculated quantity that is based on measured values has some error.
d)
The magnitude of the difference between the true value of the quantity and the individual measurement value is called the relative error of the measurement.
|
|
Raghav Bansal answered |
The magnitude of the difference between the true value of the quantity and the individual measurement value is called the absolute error of the measurement. Hence, option (d) is an incorrect statement while all other statements are correct.
Which of the following is not unit of length?- a)angstrom
- b)fermi
- c)barn
- d)parsec
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not unit of length?
a)
angstrom
b)
fermi
c)
barn
d)
parsec
|
|
Suresh Iyer answered |
Angstrom, femi and parsec are the units of length whereas barn is the unit of nuclear cross-section.
The Sun's angular diameter is measured to be 1920". The distance of the sun from the earth is 1.496 × 1011m. What is the diameter of the sun?- a)1.39 × 109 m
- b)1.39 × 1010 m
- c)1.39 × 1011 m
- d)1.39 × 1012 m
Correct answer is option 'A'. Can you explain this answer?
The Sun's angular diameter is measured to be 1920". The distance of the sun from the earth is 1.496 × 1011m. What is the diameter of the sun?
a)
1.39 × 109 m
b)
1.39 × 1010 m
c)
1.39 × 1011 m
d)
1.39 × 1012 m
|
|
Siddharth Chavan answered |
The angular diameter of the Sun is observed to be 1920 arcseconds.
The device used for measuring the mass of atoms and molecules is- a)spring balance
- b)torsional balance
- c)mass spectrograph
- d)common balance
Correct answer is option 'C'. Can you explain this answer?
The device used for measuring the mass of atoms and molecules is
a)
spring balance
b)
torsional balance
c)
mass spectrograph
d)
common balance
|
|
Garima Roy answered |
Mass Spectrograph for Measuring the Mass of Atoms and Molecules
Mass spectrograph is a device used for measuring the mass of atoms and molecules. It is an important tool in analytical chemistry and is used in a wide range of applications, including chemical analysis, biomedical research, and environmental monitoring.
Principle of Mass Spectrograph
The principle of mass spectrograph is based on the separation of ions according to their mass and charge. The sample is ionized by bombarding it with high-energy electrons, causing the atoms or molecules to lose one or more electrons and become positively charged ions. These ions are then accelerated by an electric field and passed through a magnetic field. The magnetic field causes the ions to follow a curved path, with the degree of curvature depending on the mass-to-charge ratio of the ion. The ions are detected by a detector, which generates a signal that is proportional to the number of ions of a particular mass-to-charge ratio.
Components of Mass Spectrograph
- Ion source: The ion source is used to ionize the sample and produce a beam of ions. There are several types of ion sources, including electron impact ionization, chemical ionization, and electrospray ionization.
- Accelerator: The accelerator is used to accelerate the ions to a high velocity. This is typically done using an electric field.
- Analyzer: The analyzer is used to separate the ions according to their mass-to-charge ratio. There are several types of analyzers, including magnetic sector analyzers, time-of-flight analyzers, and quadrupole analyzers.
- Detector: The detector is used to detect the ions that have passed through the analyzer. There are several types of detectors, including Faraday cups, electron multipliers, and photomultiplier tubes.
Applications of Mass Spectrograph
Mass spectrograph is used in a wide range of applications, including:
- Chemical analysis: Mass spectrograph is used to identify the components of a sample and determine their relative abundances.
- Biomedical research: Mass spectrograph is used to study the structure and function of proteins, peptides, and other biomolecules.
- Environmental monitoring: Mass spectrograph is used to monitor the levels of pollutants in the air, water, and soil.
Conclusion
Mass spectrograph is an important tool for measuring the mass of atoms and molecules. It is based on the separation of ions according to their mass and charge and is used in a wide range of applications, including chemical analysis, biomedical research, and environmental monitoring.
Mass spectrograph is a device used for measuring the mass of atoms and molecules. It is an important tool in analytical chemistry and is used in a wide range of applications, including chemical analysis, biomedical research, and environmental monitoring.
Principle of Mass Spectrograph
The principle of mass spectrograph is based on the separation of ions according to their mass and charge. The sample is ionized by bombarding it with high-energy electrons, causing the atoms or molecules to lose one or more electrons and become positively charged ions. These ions are then accelerated by an electric field and passed through a magnetic field. The magnetic field causes the ions to follow a curved path, with the degree of curvature depending on the mass-to-charge ratio of the ion. The ions are detected by a detector, which generates a signal that is proportional to the number of ions of a particular mass-to-charge ratio.
Components of Mass Spectrograph
- Ion source: The ion source is used to ionize the sample and produce a beam of ions. There are several types of ion sources, including electron impact ionization, chemical ionization, and electrospray ionization.
- Accelerator: The accelerator is used to accelerate the ions to a high velocity. This is typically done using an electric field.
- Analyzer: The analyzer is used to separate the ions according to their mass-to-charge ratio. There are several types of analyzers, including magnetic sector analyzers, time-of-flight analyzers, and quadrupole analyzers.
- Detector: The detector is used to detect the ions that have passed through the analyzer. There are several types of detectors, including Faraday cups, electron multipliers, and photomultiplier tubes.
Applications of Mass Spectrograph
Mass spectrograph is used in a wide range of applications, including:
- Chemical analysis: Mass spectrograph is used to identify the components of a sample and determine their relative abundances.
- Biomedical research: Mass spectrograph is used to study the structure and function of proteins, peptides, and other biomolecules.
- Environmental monitoring: Mass spectrograph is used to monitor the levels of pollutants in the air, water, and soil.
Conclusion
Mass spectrograph is an important tool for measuring the mass of atoms and molecules. It is based on the separation of ions according to their mass and charge and is used in a wide range of applications, including chemical analysis, biomedical research, and environmental monitoring.
Two resistors of resistances R1 = (300 ± 3)Ω and R2 = (500 ± 4)Ω are connected in series. The equivalent resistance of the series combination is- a)(800 ± 1)Ω
- b)(800 ± 7) Ω
- c)(200 ± 7) Ω
- d)(200 ± 1) Ω
Correct answer is option 'B'. Can you explain this answer?
Two resistors of resistances R1 = (300 ± 3)Ω and R2 = (500 ± 4)Ω are connected in series. The equivalent resistance of the series combination is
a)
(800 ± 1)Ω
b)
(800 ± 7) Ω
c)
(200 ± 7) Ω
d)
(200 ± 1) Ω
|
|
Jyoti Sengupta answered |
The equivalent resistance of series combination is
Rs = R1 + R2 = 300Ω + 500Ω = 800Ω
The error in equivalent resistance is given by
ΔR = (ΔR1 + ΔR2) = (3 + 4)Ω = 7Ω
Hence, the equivalent resistance along with error is (800 ± 7)Ω.
Rs = R1 + R2 = 300Ω + 500Ω = 800Ω
The error in equivalent resistance is given by
ΔR = (ΔR1 + ΔR2) = (3 + 4)Ω = 7Ω
Hence, the equivalent resistance along with error is (800 ± 7)Ω.
Which of the following physical quantities has neither units nor dimensions?- a)Relative velocity
- b)Relative density
- c)Angle
- d)Energy
Correct answer is option 'B'. Can you explain this answer?
Which of the following physical quantities has neither units nor dimensions?
a)
Relative velocity
b)
Relative density
c)
Angle
d)
Energy
|
|
Dev Patel answered |
Relative density is the ratio of two like quantities.
Therefore, it has neither unit nor dimensions.
Therefore, it has neither unit nor dimensions.
In International System of units, there are seven base quantities whose units are defined. Which physical quantity has a prefix with its unit?
- a)Mass
- b)Thermodynamic temperature
- c)Luminous intensity
- d)Amount of substance
Correct answer is option 'A'. Can you explain this answer?
In International System of units, there are seven base quantities whose units are defined. Which physical quantity has a prefix with its unit?
a)
Mass
b)
Thermodynamic temperature
c)
Luminous intensity
d)
Amount of substance
|
|
Preeti Iyer answered |
The SI unit of mass is kilogram in which 'kilo' is the prefix.
The number of significant figures in the numbers 4.8000 x 104 and 48000.50 are respectively- a)5 and 6
- b)5 and 7
- c)2 and 7
- d)2 and 6
Correct answer is option 'B'. Can you explain this answer?
The number of significant figures in the numbers 4.8000 x 104 and 48000.50 are respectively
a)
5 and 6
b)
5 and 7
c)
2 and 7
d)
2 and 6
|
|
Ritika Reddy answered |
Significant figures refer to the number of digits in a number that have meaning or contribute to the accuracy of the number. Here, we need to determine the number of significant figures in the numbers 4.8000 x 104 and 48000.50.
4.8000 x 104:
- There are five digits after the decimal point, but trailing zeros after a decimal point are not significant.
- The number 4.8000 has five significant figures because all the zeros between the non-zero digits are significant.
- The exponent 104 indicates that the decimal point is shifted 4 places to the right, so there are 4 additional significant figures.
- Therefore, the total number of significant figures is 5 + 4 = 9.
48000.50:
- There are six digits before the decimal point and two digits after the decimal point.
- All the digits are non-zero, so they are all significant.
- Therefore, the total number of significant figures is 6 + 2 = 8.
Therefore, the correct answer is option B - 5 and 7.
4.8000 x 104:
- There are five digits after the decimal point, but trailing zeros after a decimal point are not significant.
- The number 4.8000 has five significant figures because all the zeros between the non-zero digits are significant.
- The exponent 104 indicates that the decimal point is shifted 4 places to the right, so there are 4 additional significant figures.
- Therefore, the total number of significant figures is 5 + 4 = 9.
48000.50:
- There are six digits before the decimal point and two digits after the decimal point.
- All the digits are non-zero, so they are all significant.
- Therefore, the total number of significant figures is 6 + 2 = 8.
Therefore, the correct answer is option B - 5 and 7.
The radius of a sphere is 1.41cm. Its volume to an appropriate number of significant figures is- a)11.73 cm3
- b)11.736 cm3
- c)11.7 cm3
- d)117 cm3
Correct answer is option 'C'. Can you explain this answer?
The radius of a sphere is 1.41cm. Its volume to an appropriate number of significant figures is
a)
11.73 cm3
b)
11.736 cm3
c)
11.7 cm3
d)
117 cm3
|
|
Preeti Iyer answered |
Radius of the sphere, r = 1.41cm
(3 significant figures)
Volume of the sphere,
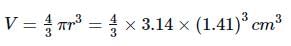
(3 significant figures)
Volume of the sphere,
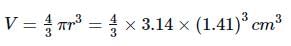
= 11.736 cm3
Rounded off upto 3 significant figures = 11.7 cm3.
Rounded off upto 3 significant figures = 11.7 cm3.
The value of universal gravitational constant G = 6.67 × 10−11 N m2 kg−2. The value of G in units of g−1 cm3 s−2 is- a)6.67 × 10−8
- b)6.67 × 10−7
- c)6.67 × 10−9
- d)6.67 × 10−10
Correct answer is option 'A'. Can you explain this answer?
The value of universal gravitational constant G = 6.67 × 10−11 N m2 kg−2. The value of G in units of g−1 cm3 s−2 is
a)
6.67 × 10−8
b)
6.67 × 10−7
c)
6.67 × 10−9
d)
6.67 × 10−10
|
|
Mira Joshi answered |
G = 6.67 × 10−11 N m2 kg−2
= 6.67 × 10−11 × (kg m s−2)(m2)(kg)−2
= 6.67 × 10−11 × [(1000 g) × (100 cm) × s−2] × (100 cm)2 × (1000 g)−2
= 6.67 × 10−8 g−1 cm3 s−2
= 6.67 × 10−11 × (kg m s−2)(m2)(kg)−2
= 6.67 × 10−11 × [(1000 g) × (100 cm) × s−2] × (100 cm)2 × (1000 g)−2
= 6.67 × 10−8 g−1 cm3 s−2
Which of the following is the most precise instrument for measuring length?- a)Metre rod of least count 0.1cm
- b)Vernier callipers of least count 0.01cm
- c)Screw gauge of least count 0.001cm
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following is the most precise instrument for measuring length?
a)
Metre rod of least count 0.1cm
b)
Vernier callipers of least count 0.01cm
c)
Screw gauge of least count 0.001cm
d)
None of these
|
|
Rohit Das answered |
Precision in Measuring Length
Measuring length is one of the fundamental aspects of physics and engineering. The accuracy and precision of any measurement depend on the instrument used to take the measurement. Precision refers to the degree of exactness with which a measurement is made. The most precise instrument for measuring length is the one that has the smallest least count.
Definition of Least Count
Least count refers to the smallest measurement that an instrument can make. For example, if the least count of a measuring instrument is 0.1 cm, then the instrument can measure lengths that are multiples of 0.1 cm. The smaller the least count, the more precise the instrument.
Comparison of Instruments
a) Metre Rod of Least Count 0.1 cm
A metre rod is a simple instrument used to measure length. It is a straight rod that is one metre in length. The least count of a metre rod is usually 0.1 cm. This means that the rod can measure lengths that are multiples of 0.1 cm. However, the metre rod is not a very precise instrument for measuring length.
b) Vernier Callipers of Least Count 0.01 cm
A vernier caliper is a more precise instrument for measuring length. It consists of two jaws, an upper and a lower, that can be adjusted to fit the object being measured. The jaws are connected to a scale that can be read to the nearest 0.1 mm. The least count of a vernier caliper is 0.01 cm, which means that it can measure lengths that are multiples of 0.01 cm.
c) Screw Gauge of Least Count 0.001 cm
A screw gauge is the most precise instrument for measuring length. It consists of a U-shaped frame with a screw attached to one end. The screw has a scale that can be read to the nearest 0.01 mm. The least count of a screw gauge is 0.001 cm, which means that it can measure lengths that are multiples of 0.001 cm.
Conclusion
In conclusion, the screw gauge is the most precise instrument for measuring length because it has the smallest least count. The smaller the least count, the more precise the instrument. While a metre rod and vernier calipers can also be used to measure length, they are not as precise as a screw gauge.
Measuring length is one of the fundamental aspects of physics and engineering. The accuracy and precision of any measurement depend on the instrument used to take the measurement. Precision refers to the degree of exactness with which a measurement is made. The most precise instrument for measuring length is the one that has the smallest least count.
Definition of Least Count
Least count refers to the smallest measurement that an instrument can make. For example, if the least count of a measuring instrument is 0.1 cm, then the instrument can measure lengths that are multiples of 0.1 cm. The smaller the least count, the more precise the instrument.
Comparison of Instruments
a) Metre Rod of Least Count 0.1 cm
A metre rod is a simple instrument used to measure length. It is a straight rod that is one metre in length. The least count of a metre rod is usually 0.1 cm. This means that the rod can measure lengths that are multiples of 0.1 cm. However, the metre rod is not a very precise instrument for measuring length.
b) Vernier Callipers of Least Count 0.01 cm
A vernier caliper is a more precise instrument for measuring length. It consists of two jaws, an upper and a lower, that can be adjusted to fit the object being measured. The jaws are connected to a scale that can be read to the nearest 0.1 mm. The least count of a vernier caliper is 0.01 cm, which means that it can measure lengths that are multiples of 0.01 cm.
c) Screw Gauge of Least Count 0.001 cm
A screw gauge is the most precise instrument for measuring length. It consists of a U-shaped frame with a screw attached to one end. The screw has a scale that can be read to the nearest 0.01 mm. The least count of a screw gauge is 0.001 cm, which means that it can measure lengths that are multiples of 0.001 cm.
Conclusion
In conclusion, the screw gauge is the most precise instrument for measuring length because it has the smallest least count. The smaller the least count, the more precise the instrument. While a metre rod and vernier calipers can also be used to measure length, they are not as precise as a screw gauge.
The SI unit of pressure gradient is- a)N m-2
- b)N m
- c)N m-1
- d)N m-3
Correct answer is option 'D'. Can you explain this answer?
The SI unit of pressure gradient is
a)
N m-2
b)
N m
c)
N m-1
d)
N m-3
|
|
Ameya Sharma answered |
SI unit of pressure gradient:
The correct answer is option 'D': N m-3.
Explanation:
Pressure gradient:
The pressure gradient is a measure of the change in pressure over a given distance. It represents how quickly the pressure is changing in a particular direction.
SI unit of pressure:
The SI unit of pressure is the Pascal (Pa), which is defined as one Newton per square meter (N m-2).
Derivation of SI unit of pressure gradient:
To derive the SI unit of pressure gradient, we need to consider the definition of pressure gradient.
Pressure gradient = Change in pressure / Distance
The change in pressure is measured in Pascal (Pa), and the distance is measured in meters (m). Therefore, the SI unit of pressure gradient can be determined by dividing the unit of pressure by the unit of distance.
SI unit of pressure gradient = Pascal / meter
Since Pascal is equivalent to one Newton per square meter (N m-2), the SI unit of pressure gradient can be further simplified as:
SI unit of pressure gradient = (N m-2) / meter
Simplifying further:
SI unit of pressure gradient = N m-2 m-1
Using the laws of exponents:
SI unit of pressure gradient = N m-3
Therefore, the SI unit of pressure gradient is N m-3.
Summary:
The SI unit of pressure gradient is N m-3, which means Newton per cubic meter. It represents the change in pressure per unit volume and is derived by dividing the unit of pressure (Pascal) by the unit of distance (meter).
The correct answer is option 'D': N m-3.
Explanation:
Pressure gradient:
The pressure gradient is a measure of the change in pressure over a given distance. It represents how quickly the pressure is changing in a particular direction.
SI unit of pressure:
The SI unit of pressure is the Pascal (Pa), which is defined as one Newton per square meter (N m-2).
Derivation of SI unit of pressure gradient:
To derive the SI unit of pressure gradient, we need to consider the definition of pressure gradient.
Pressure gradient = Change in pressure / Distance
The change in pressure is measured in Pascal (Pa), and the distance is measured in meters (m). Therefore, the SI unit of pressure gradient can be determined by dividing the unit of pressure by the unit of distance.
SI unit of pressure gradient = Pascal / meter
Since Pascal is equivalent to one Newton per square meter (N m-2), the SI unit of pressure gradient can be further simplified as:
SI unit of pressure gradient = (N m-2) / meter
Simplifying further:
SI unit of pressure gradient = N m-2 m-1
Using the laws of exponents:
SI unit of pressure gradient = N m-3
Therefore, the SI unit of pressure gradient is N m-3.
Summary:
The SI unit of pressure gradient is N m-3, which means Newton per cubic meter. It represents the change in pressure per unit volume and is derived by dividing the unit of pressure (Pascal) by the unit of distance (meter).
Chapter doubts & questions for Units and Measurements - NCERT Based Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Units and Measurements - NCERT Based Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup