All Exams >
Class 10 >
Online MCQ Tests for Class 10 >
All Questions
All questions of Chemical Reactions and Equations for Class 10 Exam
Which of the following is a combination reaction?
- a)CaCO3 → CaO + CO2
- b)H2 + Cl2 → 2HCl
- c)H2CO3 → H2O + CO2
- d)2KClO3 → 2KCl + 3O2
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a combination reaction?
a)
CaCO3 → CaO + CO2
b)
H2 + Cl2 → 2HCl
c)
H2CO3 → H2O + CO2
d)
2KClO3 → 2KCl + 3O2
|
|
Roshni chauhan answered |
Balanced Equation:
H2 + Cl2 → 2HCl2
H2 + Cl2 → 2HCl2
The above reaction is an example of combination reaction because two different elements are combining to form a single compound.
Which of the following is a decomposition reaction?- a)NaOH + HCl → NaCl + H2O
- b)NH4CNO → H2NCONH2
- c)2KClO3 → 2KCl + 3O2
- d)H2 + I2 → 2HI
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a decomposition reaction?
a)
NaOH + HCl → NaCl + H2O
b)
NH4CNO → H2NCONH2
c)
2KClO3 → 2KCl + 3O2
d)
H2 + I2 → 2HI
|
|
Meera Rana answered |
A decomposition reaction occurs when one reactant breaks down into two or more products. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
2KClO3 → 2KCl + 3O2
2KClO3 → 2KCl + 3O2
The reaction C + O2 → CO2 + Heat is a:
- a)Combination reaction
- b)Oxidation reaction
- c)Exothermic reaction
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
The reaction C + O2 → CO2 + Heat is a:
a)
Combination reaction
b)
Oxidation reaction
c)
Exothermic reaction
d)
All of the above
|
|
Amit Sharma answered |
C + O2 → CO2 + Heat
- This is a Combination reaction because C and O2 are combining to produce one single compound CO2.
- This is also an Oxidation reaction because carbon is getting oxidized.
- This is also an exothermic reaction because in this reaction heat is getting released.
So Option D is correct
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?- a)Red litmus solution
- b)Lime water
- c)A burning splinter
- d)Blue litmus solution
Correct answer is option 'C'. Can you explain this answer?
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?
a)
Red litmus solution
b)
Lime water
c)
A burning splinter
d)
Blue litmus solution
|
|
Kiran Mehta answered |
A burning splinter since hydrogen gas burns with a pop sound.
Zinc being an active metal readily reacts with hydrochloric acid at room temperature to form soluble zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2 + H2.
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:- a)Decomposition reaction
- b)Combination reaction
- c)Displacement reaction
- d)Single displacement reaction
Correct answer is option 'C'. Can you explain this answer?
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
The above reaction is an example of a:
a)
Decomposition reaction
b)
Combination reaction
c)
Displacement reaction
d)
Single displacement reaction

|
Anushka Chopra answered |
The given equation is a displacement reaction in which Fe of Fe2O3 has been displaced by Al. Hence, (c) is the correct option.
Can you explain the answer of this question below:ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
- A:
A = 1, B = 1, C = 2, D = 1
- B:
A = 2, B = 2, C = 2, D = 1
- C:
A = 2, B = 1, C = 2, D = 1
- D:
none of these
The answer is b.
ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
A = 1, B = 1, C = 2, D = 1
A = 2, B = 2, C = 2, D = 1
A = 2, B = 1, C = 2, D = 1
none of these
|
|
Shweta singh answered |
2 Na + 2H2O gives 2NaOH + H2
So option B is Correct answer.
Which of the following is a displacement reaction?- a)CaCO3 → CaO + CO2
- b)CaO + 2HCl → CaCl2 + H2O
- c)Fe + CuSO4 → FeSO4 + Cu
- d)NaOH + HCl → NaCl + H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a displacement reaction?
a)
CaCO3 → CaO + CO2
b)
CaO + 2HCl → CaCl2 + H2O
c)
Fe + CuSO4 → FeSO4 + Cu
d)
NaOH + HCl → NaCl + H2O
|
|
Gaurav Kumar answered |
Fe + CuSo4 → FeSO4 + Cu
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
(a) is a decomposition reaction.
(b) and (d) are neutralisation reactions.
(b) and (d) are neutralisation reactions.
The brown gas evolved on heating of copper nitrate is- a)O2
- b)NO2
- c)N2
- d)NO
Correct answer is option 'B'. Can you explain this answer?
The brown gas evolved on heating of copper nitrate is
a)
O2
b)
NO2
c)
N2
d)
NO

|
Deepika Kumar answered |
Copper nitrate when taken in hydrated form contains water molecules. Initially on heating, copper nitrate gets dehydrated. Further heating causes the decomposition of copper nitrate to release Nitrogen Di oxide (NO2) Copper Oxide (CuO) and Oxygen(O2).
Which of the following is an example of displacement reaction?- a)4 Na+ O2 → 2 Na2O
- b)2 Cu + O2 → 2 CuO
- c)Mg + 2 HCl → MgCl2 + H2
- d)N2 + 3 H2 → 2 NH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of displacement reaction?
a)
4 Na+ O2 → 2 Na2O
b)
2 Cu + O2 → 2 CuO
c)
Mg + 2 HCl → MgCl2 + H2
d)
N2 + 3 H2 → 2 NH3

|
Orion Classes answered |
It is a single replacement reaction.
Mg + 2HCl → MgCl2 + H2
Mg + 2HCl → MgCl2 + H2
Which of the following metals comes above zinc in reactivity series?
- a)Silver
- b)Copper
- c)Aluminium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals comes above zinc in reactivity series?
a)
Silver
b)
Copper
c)
Aluminium
d)
Iron
|
|
Karthik murthy answered |
Reactivity Series:
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
2AgI(s)  2Ag(s) + I2(g) The colour of iodine is
2Ag(s) + I2(g) The colour of iodine is- a)green
- b)purple
- c)brown
- d)pink
Correct answer is option 'B'. Can you explain this answer?
2AgI(s)  2Ag(s) + I2(g) The colour of iodine is
2Ag(s) + I2(g) The colour of iodine is
 2Ag(s) + I2(g) The colour of iodine is
2Ag(s) + I2(g) The colour of iodine isa)
green
b)
purple
c)
brown
d)
pink
|
|
Aditya Shah answered |
Iodine is purple in colour.
The reaction H2 + Cl2 → 2HCl is a –- a)Decomposition reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Displacement reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction H2 + Cl2 → 2HCl is a –
a)
Decomposition reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Displacement reaction
|
|
Anjana Khatri answered |
It is a reaction in which two substances react with each other to make a single substance. Therefore, H2 + CL2 = 2HCL is an COMBINATION REACTION. It is a special case of addition reaction known as synthesis.
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
- a)SO2
- b)H2S
- c)H2O
- d)S
Correct answer is option 'B'. Can you explain this answer?
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
a)
SO2
b)
H2S
c)
H2O
d)
S
|
|
Raghav Bansal answered |
- In the reaction SO2 is getting reduced to S as oxygen is being removed.
- Similarly, H2S is oxidised to S as hydrogen is removed.
- The species getting oxidised facilitates the reduction process and is the reducing agent.
- Hence, H2S is the reducing agent and Oxidising agent is SO2.
So, option B is correct
Which one of the following processes involve chemical reactions ?- a)Storing of oxygen gas under pressure in a gas cylinder
- b)Liquefaction of air
- c)Keeping petrol in a china dish in the open
- d)Heating copper wire in presence of air at high temperature
Correct answer is option 'D'. Can you explain this answer?
Which one of the following processes involve chemical reactions ?
a)
Storing of oxygen gas under pressure in a gas cylinder
b)
Liquefaction of air
c)
Keeping petrol in a china dish in the open
d)
Heating copper wire in presence of air at high temperature

|
Srishti Mishra answered |
A reaction which causes change in chemical composition of a substance and results in the formation of new substance is known as a chemical reaction.
For example, reaction of sodium with chlorine results in the formation of sodium chloride. Since there is change in chemical composition of sodium and chlorine thus a new substance sodium chloride has formed.
Therefore, when we keep petrol in a china dish then petrol will evaporate and this reaction cannot be reversed. Therefore, it is a chemical change.
Which of the following represent a double displacement reaction?- a)2H2 + O2 → 2H2O
- b)2Mg + O2 → 2MgO
- c)AgNO3 + NaCl → AgCl
 + NaNO3
+ NaNO3 - d)H2 + Cl2 → 2HCl
Correct answer is option 'C'. Can you explain this answer?
Which of the following represent a double displacement reaction?
a)
2H2 + O2 → 2H2O
b)
2Mg + O2 → 2MgO
c)
AgNO3 + NaCl → AgCl + NaNO3
+ NaNO3
d)
H2 + Cl2 → 2HCl

|
Environment Lover answered |
Yah the correct answer is c as it follow the definition of displacement reaction
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction - a)(i) only
- b)(ii) only
- c)(iv) only
- d)(ii) and (iv)
Correct answer is option 'D'. Can you explain this answer?
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
a)
(i) only
b)
(ii) only
c)
(iv) only
d)
(ii) and (iv)
|
|
Kiran Mehta answered |
BaCl2 + (NH4)2SO4 → BaSO4 + 2NH4Cl
Above reaction is a double displacement reaction as ions (both cations and anions) of the reacting molecules are exchanged. Barium ion (Ba2+) combines with sulphate ion (SO42−) to form Barium sulphate (BaSO4) and ammonium ion (NH4+) combines with chloride ion(Cl−) ion to form ammonium chloride(NH4Cl).
The above reaction is also classified as a precipitation reaction because barium and sulphate ions combine to form precipitates of barium sulphate.
Ba2+ + SO42−→ BaSO4(s)
Above reaction is a double displacement reaction as ions (both cations and anions) of the reacting molecules are exchanged. Barium ion (Ba2+) combines with sulphate ion (SO42−) to form Barium sulphate (BaSO4) and ammonium ion (NH4+) combines with chloride ion(Cl−) ion to form ammonium chloride(NH4Cl).
The above reaction is also classified as a precipitation reaction because barium and sulphate ions combine to form precipitates of barium sulphate.
Ba2+ + SO42−→ BaSO4(s)
When lead nitrate is heated, it breaks down into lead monoxide, nitrogen dioxide and oxygen.
2Pb(NO3)2 → 2PbO + 4NO2 + O2
The reaction is an example of:- a)combination reaction
- b)decomposition reaction
- c)double decomposition reaction
- d)displacement reaction
Correct answer is option 'B'. Can you explain this answer?
When lead nitrate is heated, it breaks down into lead monoxide, nitrogen dioxide and oxygen.
2Pb(NO3)2 → 2PbO + 4NO2 + O2
The reaction is an example of:
2Pb(NO3)2 → 2PbO + 4NO2 + O2
The reaction is an example of:
a)
combination reaction
b)
decomposition reaction
c)
double decomposition reaction
d)
displacement reaction
|
|
Ananya Das answered |
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a decomposition reaction is AB→A+B. Most decomposition reactions require an input of energy in the form of heat, light, or electricity.
Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride. This is a:- a)decomposition reaction
- b)combination reaction
- c)displacement reaction
- d)double displacement reaction
Correct answer is option 'B'. Can you explain this answer?
Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride. This is a:
a)
decomposition reaction
b)
combination reaction
c)
displacement reaction
d)
double displacement reaction
|
|
Arun Sharma answered |
Reaction of magnesium with nitrogen is a combination reaction as these two reactants (Mg and N2) combine to form a single product as magnesium nitride (Mg3N2). The equation can be written as: 3Mg(s) + N2(g) → Mg3N2(s).
What is the number of iron atoms and hydrogen atoms in the balanced equation for the reaction of steam on iron ?- a)3Fe and 8H
- b)3Fe and 4H
- c)3Fe and 6H
- d)3Fe and 10H
Correct answer is option 'B'. Can you explain this answer?
What is the number of iron atoms and hydrogen atoms in the balanced equation for the reaction of steam on iron ?
a)
3Fe and 8H
b)
3Fe and 4H
c)
3Fe and 6H
d)
3Fe and 10H
|
|
Amit Kumar answered |
The balanced equation for the reaction is
3Fe + 4H2O → Fe3O4 + 4H2
3Fe + 4H2O → Fe3O4 + 4H2
Which of the following gases can be used for storage of fresh sample of an oil for a long time?- a)Carbon dioxide or oxygen
- b)Nitrogen or oxygen
- c)Carbon dioxide or helium
- d)Helium or nitrogen
Correct answer is option 'D'. Can you explain this answer?
Which of the following gases can be used for storage of fresh sample of an oil for a long time?
a)
Carbon dioxide or oxygen
b)
Nitrogen or oxygen
c)
Carbon dioxide or helium
d)
Helium or nitrogen
|
|
Ananya Das answered |
Nitrogen or helium can be used for storage of fresh sample of oil for long time. Helium and Nitrogen both the gases provide inert atmosphere.
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?- a)2H2(I) + O2(I) → 2H2O (g)
- b)2H2(g) + O2(I) → 2H2O (I)
- c)2H2(g) + O2(g) → 2H2O (I)
- d)2H2(g) + O2(g) → 2H2O (g)
Correct answer is option 'C'. Can you explain this answer?
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
a)
2H2(I) + O2(I) → 2H2O (g)
b)
2H2(g) + O2(I) → 2H2O (I)
c)
2H2(g) + O2(g) → 2H2O (I)
d)
2H2(g) + O2(g) → 2H2O (g)
|
|
Amit Sharma answered |
As we know, H2 and O2 are present in the form of gas in atmosphere and H2O as liquid.
The colour of zinc metal is
- a)reddish-brown
- b)grey
- c)Blue
- d)silvery-white
Correct answer is option 'D'. Can you explain this answer?
The colour of zinc metal is
a)
reddish-brown
b)
grey
c)
Blue
d)
silvery-white
|
|
Vikas Kumar answered |
The correct option is D
silvery-white
Explanation for correct option
(D) The color of Zinc metal is silvery.
- An electron gets excited to a higher energy orbital from a lower energy d orbital. The frequency of light absorbed is proportional to the excitation energy.
- This frequency is usually in the visible range.
- The color seen corresponds to the complementary color of the light absorbed.
- Zinc (Zn) is a silvery-white metal.
Hence, option (D) is correct. The color of Zinc metal is silvery.
Which of the following is not an example of chemical change?- a)rusting of iron
- b)milk changes to curd
- c)digestion of food in our body
- d)changing of water to water vapour
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an example of chemical change?
a)
rusting of iron
b)
milk changes to curd
c)
digestion of food in our body
d)
changing of water to water vapour
|
|
Amit Sharma answered |
The process of changing water vapour into water is called condensation. It is not a chemical reaction.
When milk converts in curd the process is irreversible and a new substance with different properties is formed hence it is an example of a chemical change.
Rusting of iron is also a chemical change.
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed ?
(i) It is an endothermic reaction.
(ii) It is an exothermic reaction.
(iii) The pH of the resulting solution will be more than seven.
(iv) The pH of the resulting solution will be less than seven.- a)(i) and (ii)
- b)(ii) and (iii)
- c)(i) and (iv)
- d)(iii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed ?
(i) It is an endothermic reaction.
(ii) It is an exothermic reaction.
(iii) The pH of the resulting solution will be more than seven.
(iv) The pH of the resulting solution will be less than seven.
(i) It is an endothermic reaction.
(ii) It is an exothermic reaction.
(iii) The pH of the resulting solution will be more than seven.
(iv) The pH of the resulting solution will be less than seven.
a)
(i) and (ii)
b)
(ii) and (iii)
c)
(i) and (iv)
d)
(iii) and (iv)
|
|
Amit Kumar answered |
Quick lime(CaO) combines with water to form calcium hydroxide (Ca(OH)2). It is an exothermic reaction as heat is produced in the reaction.
CaO + H2O → Ca(OH)2 + heat
On adding excess of calcium hydroxide in water, a saturated solution of calcium hydroxide is formed which is called lime water. Lime water is basic in nature and therefore has a pH above 7.
CaO + H2O → Ca(OH)2 + heat
On adding excess of calcium hydroxide in water, a saturated solution of calcium hydroxide is formed which is called lime water. Lime water is basic in nature and therefore has a pH above 7.
Which of the following are exothermic reactions?
(i) Burning of coal.
(ii) Reaction of water with quicklime.
(iii) Respiration.
(iv) Evaporation of water.
- a)(i), (ii) and (iv)
- b)(i) and (ii) only
- c)(ii) and (iii) only
- d)(i), (ii) and (iii)
Correct answer is option 'D'. Can you explain this answer?
Which of the following are exothermic reactions?
(i) Burning of coal.
(ii) Reaction of water with quicklime.
(iii) Respiration.
(iv) Evaporation of water.
a)
(i), (ii) and (iv)
b)
(i) and (ii) only
c)
(ii) and (iii) only
d)
(i), (ii) and (iii)
|
|
Vikram Kapoor answered |
1)Burning of coal undergoes combustion reaction. Combustion reaction is a type of reaction in which chemical species experiences oxidation by undergoing a reaction with the oxidizing agent which results in the emission of energy in the form of heat. It is an exothermic process.
2)Quick lime is calcium oxide. It reacts with water to form calcium hydroxide. The reaction of water with quicklime releases a large amount of energy. Hence, this is an exothermic reaction.
3) Respiration is a series of exothermic reactions that occur in the mitochondria of living cells in order to release energy from food molecules. This energy can then be used to produce heat, for movement, growth, reproduction and active uptake.
4)
Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy.Only evaporation of water is an endothermic reaction.
Mark the incorrect statement :- a)Substances appearing on the left hand side of the equation are called products
- b)Substances appearing on the right hand side of the equation are called products
- c)Substances appearing on the left hand side of the equation are called reactants
- d)Reactants and products are separated by an arrow mark
Correct answer is option 'A'. Can you explain this answer?
Mark the incorrect statement :
a)
Substances appearing on the left hand side of the equation are called products
b)
Substances appearing on the right hand side of the equation are called products
c)
Substances appearing on the left hand side of the equation are called reactants
d)
Reactants and products are separated by an arrow mark
|
|
Amit Sharma answered |
“The statement that substances appearing on the left hand side of the equation are called products” is incorrect.
The following reaction is used for the preparation of oxygen gas in the laboratory
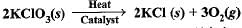
Which of the following statement (s) is (are) correct about the reaction ?- a)It is a decomposition reaction and endothermic in nature
- b)It is a combination reaction
- c)It is a decomposition reaction and accompanied by release of heat
- d)It is a photochemical decomposition reaction and exothermic in nature
Correct answer is option 'A'. Can you explain this answer?
The following reaction is used for the preparation of oxygen gas in the laboratory
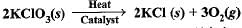
Which of the following statement (s) is (are) correct about the reaction ?
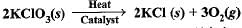
Which of the following statement (s) is (are) correct about the reaction ?
a)
It is a decomposition reaction and endothermic in nature
b)
It is a combination reaction
c)
It is a decomposition reaction and accompanied by release of heat
d)
It is a photochemical decomposition reaction and exothermic in nature
|
|
Krishna Iyer answered |
Clearly, It is a decomposition reaction and it requires heat to get decomposed into KCl and O2.
When magnesium ribbon is burnt in air, the ash formed is- a)white
- b)green
- c)yellow
- d)black
Correct answer is option 'A'. Can you explain this answer?
When magnesium ribbon is burnt in air, the ash formed is
a)
white
b)
green
c)
yellow
d)
black
|
|
Anita Menon answered |
Ash is white powder of magnesium oxide.
The reaction :Fe2O3 + 2Al → Al2O3 + 2Fe is an example of- a)decomposition reaction
- b)displacement reaction
- c)combination reaction
- d)double displacement reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction :
Fe2O3 + 2Al → Al2O3 + 2Fe is an example of
a)
decomposition reaction
b)
displacement reaction
c)
combination reaction
d)
double displacement reaction
|
|
Pooja Shah answered |
In the chemical reaction Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is known as displacement reaction iron oxide reacts with aluminium to form aluminium oxide and iron metal.
Which among the following is (are) double displacement reaction(s) ?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 →CO2 + 2H2O- a)(i) and (iv)
- b)(ii) only
- c)(i) and (ii)
- d)(iii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
Which among the following is (are) double displacement reaction(s) ?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 →CO2 + 2H2O
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 →CO2 + 2H2O
a)
(i) and (iv)
b)
(ii) only
c)
(i) and (ii)
d)
(iii) and (iv)
|
|
Vivek Rana answered |
The anions and cations are exchanged in the reaction (ii) only.
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:- a)Displacement reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Decomposition reaction
Correct answer is option 'A'. Can you explain this answer?
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:
a)
Displacement reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Decomposition reaction
|
|
Neha Patel answered |
This is a metal/acid reaction if carried out in aqueous solution.
It is also a displacement reaction where 1 atom of Zinc displaces 2 H+ ions
and it is a redox reaction as the Zn atom loses electrons (it is OXIDISED) and the 2 H+ ions gain electrons (they are REDUCED)
What happens when dil hydrochloric acid is added to iron fillings?- a)Hydrogen gas and Iron chloride are produced
- b)Chlorine gas and Iron hydroxide are produced
- c)NO reaction takes place
- d)Iron salt and water are produced
Correct answer is option 'A'. Can you explain this answer?
What happens when dil hydrochloric acid is added to iron fillings?
a)
Hydrogen gas and Iron chloride are produced
b)
Chlorine gas and Iron hydroxide are produced
c)
NO reaction takes place
d)
Iron salt and water are produced
|
|
Amit Sharma answered |
- Hydrogen gas and iron chloride are produced.
Fe + HCl → FeCl2 + H2
- The iron displaces hydrogen from hydrochloric acid to form iron (II) chloride & hydrogen gas. This is a single displacement reaction.
- Thus the answer is option (A) Hydrogen gas and iron chloride are produced.
In the reaction,SO2(g) + 2H2S(g) → 2H2O(l) + S (s)the reducing agent is- a)SO2
- b)H2O
- c)H2S
- d)S
Correct answer is option 'C'. Can you explain this answer?
In the reaction,
SO2(g) + 2H2S(g) → 2H2O(l) + S (s)
the reducing agent is
a)
SO2
b)
H2O
c)
H2S
d)
S
|
|
Radha Iyer answered |
H2S gets oxidised and hence it is a reducing agent.
Rahul took some zinc granules in a test tube and added dilute HCl to it. He observed that the colour of zinc granules changed to- a)yellow
- b)brown
- c)black
- d)white
Correct answer is option 'C'. Can you explain this answer?
Rahul took some zinc granules in a test tube and added dilute HCl to it. He observed that the colour of zinc granules changed to
a)
yellow
b)
brown
c)
black
d)
white
|
|
Radha nair answered |
Explanation:
When zinc granules are added to dilute hydrochloric acid (HCl), a chemical reaction takes place. The zinc reacts with the hydrogen ions in the acid to produce zinc chloride and hydrogen gas. This reaction is known as a single displacement reaction, where one element (zinc) displaces another element (hydrogen) from its compound (HCl). The reaction can be represented as follows:
Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
Now, let's look at the colour change of the zinc granules:
Therefore, the correct answer is option C (black). However, it is important to note that the colour change observed may vary depending on the purity of the zinc granules and the conditions under which the reaction takes place.
When zinc granules are added to dilute hydrochloric acid (HCl), a chemical reaction takes place. The zinc reacts with the hydrogen ions in the acid to produce zinc chloride and hydrogen gas. This reaction is known as a single displacement reaction, where one element (zinc) displaces another element (hydrogen) from its compound (HCl). The reaction can be represented as follows:
Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
Now, let's look at the colour change of the zinc granules:
- Yellow: Zinc metal is typically shiny and silvery in colour. When exposed to air, it can form a thin layer of zinc oxide on its surface, which appears as a dull yellow colour. However, this is not the colour change observed in this reaction.
- Brown: A brown colour could indicate the presence of impurities or contaminants in the zinc granules. However, this is not a typical colour change observed in this reaction.
- Black: When the reaction takes place, the zinc granules react with the HCl to form zinc chloride, which is a white solid. However, if the zinc granules are not fully covered by the acid, they may react with the oxygen in the air to form zinc oxide, which appears as a black colour. Therefore, the black colour observed in this reaction may be due to the formation of zinc oxide on the surface of the zinc granules.
- White: Zinc chloride is a white solid, and it is the product formed in this reaction. Therefore, the most likely colour change observed in this reaction is a change from silver or yellowish to white due to the formation of zinc chloride.
Therefore, the correct answer is option C (black). However, it is important to note that the colour change observed may vary depending on the purity of the zinc granules and the conditions under which the reaction takes place.
An element X on exposure to moist air turns reddish-brown and a new compound Y is formed. The substance X and Y are- a)X = Fe, Y = Fe2O3
- b)X = Ag, Y = Ag2S
- c)X = Cu, Y = CuO
- d)X = Al, Y = Al2O3
Correct answer is option 'A'. Can you explain this answer?
An element X on exposure to moist air turns reddish-brown and a new compound Y is formed. The substance X and Y are
a)
X = Fe, Y = Fe2O3
b)
X = Ag, Y = Ag2S
c)
X = Cu, Y = CuO
d)
X = Al, Y = Al2O3
|
|
Suresh Reddy answered |
An element X exposure to moist air, forms a reddish brown and a new compound Y is formed.
Fe + H2O +O2 → Fe2 O3
This Fe2O3 is the reddish brown compound which is called rust.
So , X → Fe
Y → Fe2O3
Fe + H2O +O2 → Fe2 O3
This Fe2O3 is the reddish brown compound which is called rust.
So , X → Fe
Y → Fe2O3
So Option A is correct
Which of the following is (are) an endothermic process(es) ?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water- a)(i) and (iii)
- b)(ii) only
- c)(iii) only
- d)(ii) and (iv)
Correct answer is option 'D'. Can you explain this answer?
Which of the following is (are) an endothermic process(es) ?
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
(i) Dilution of sulphuric acid
(ii) Sublimation of dry ice
(iii) Condensation of water vapours
(iv) Evaporation of water
a)
(i) and (iii)
b)
(ii) only
c)
(iii) only
d)
(ii) and (iv)
|
|
Anita Menon answered |
Heat is absorbed when dry ice sublimes and water evaporates. Therefore, cooling is produced.
In the reaction3MnO2 + 4Al → 3Mn + 2Al2O3the oxidising agent is- a)MnO2
- b)Al
- c)Al2O3
- d)Mn
Correct answer is option 'A'. Can you explain this answer?
In the reaction
3MnO2 + 4Al → 3Mn + 2Al2O3
the oxidising agent is
a)
MnO2
b)
Al
c)
Al2O3
d)
Mn
|
|
Radha Iyer answered |
MnO2 loses oxygen and therefore, gets reduced. Hence, it acts as an oxidising agent.
The process of reduction involves- a)removal of hydrogen
- b)gain of electrons
- c)addition of oxygen
- d)loss of electrons
Correct answer is option 'B'. Can you explain this answer?
The process of reduction involves
a)
removal of hydrogen
b)
gain of electrons
c)
addition of oxygen
d)
loss of electrons
|
|
Amit Sharma answered |
The process of reduction involves gain of electrons.
A student placed a Zn rod in the FeSO4 solution. After 10 hours when the rod was taken out and it was observed that:- a)Zn rod became thinner
- b)Zn rod became thicker due to iron deposition
- c)Zn rod remains as it was
- d)Zn rod has holes
Correct answer is option 'D'. Can you explain this answer?
A student placed a Zn rod in the FeSO4 solution. After 10 hours when the rod was taken out and it was observed that:
a)
Zn rod became thinner
b)
Zn rod became thicker due to iron deposition
c)
Zn rod remains as it was
d)
Zn rod has holes
|
|
Mia answered |

Electrolysis of water is a decomposition reaction. The molar ratio of hydrogen and oxygen gases liberated during electrolysis of water is- a)1 : 1
- b)2 : 1
- c)4 : 1
- d)1 : 2
Correct answer is option 'B'. Can you explain this answer?
Electrolysis of water is a decomposition reaction. The molar ratio of hydrogen and oxygen gases liberated during electrolysis of water is
a)
1 : 1
b)
2 : 1
c)
4 : 1
d)
1 : 2
|
|
Ishan Choudhury answered |
The water decomposes during electrolysis to form hydrogen and oxygen gases in the ratio 2:1 by volume.
Iron sulphate crystals on heating show the evolution of gas which- a)turns lime water milky
- b)makes a burning matchstick burn more brightly
- c)burns with a pop sound
- d)causes choking and suffocation
Correct answer is option 'D'. Can you explain this answer?
Iron sulphate crystals on heating show the evolution of gas which
a)
turns lime water milky
b)
makes a burning matchstick burn more brightly
c)
burns with a pop sound
d)
causes choking and suffocation
|
|
Avinash Patel answered |
SO2 and SO3 gases are evolved which cause choking and suffocation.
H2SO4 + 2NaOH → Na2SO4 + 2H2O Above equation is a(i) neutralization reaction(ii) double displacement reaction(iii) decomposition reaction(iv) addition reaction- a)(iii) and (iv)
- b)(i) and (ii)
- c)(i) and (iii)
- d)(ii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
H2SO4 + 2NaOH → Na2SO4 + 2H2O Above equation is a
(i) neutralization reaction
(ii) double displacement reaction
(iii) decomposition reaction
(iv) addition reaction
a)
(iii) and (iv)
b)
(i) and (ii)
c)
(i) and (iii)
d)
(ii) and (iv)

|
Saxena Aastha answered |
This is both double displacement as well as neutralization
as H2SO4 is acid and NaOH is base also they are displacing each other and forming respective compound so correct answer is B
On heating crystals of ferrous sulphate, products obtained are:- a)Ferric oxide, Sulphur dioxide, Sulphur trioxide
- b)Ferric oxide, Ferrous sulphide, Oxygen
- c)Ferrous sulphide, Sulphur dioxide, Oxygen
- d)Ferric oxide, Sulphur trioxide, Oxygen
Correct answer is option 'A'. Can you explain this answer?
On heating crystals of ferrous sulphate, products obtained are:
a)
Ferric oxide, Sulphur dioxide, Sulphur trioxide
b)
Ferric oxide, Ferrous sulphide, Oxygen
c)
Ferrous sulphide, Sulphur dioxide, Oxygen
d)
Ferric oxide, Sulphur trioxide, Oxygen
|
|
Krishna Iyer answered |
On heating, ferrous sulphate crystals lose water and anhydrous ferrous sulphate (FeSO4) is formed. So their colour changes from light green to white. On further heating, anhydrous ferrous sulphate decomposes to form ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
Which is the reducing agent in the reaction ?ZnO + C → Zn + CO- a)C
- b)Zn
- c)CO
- d)ZnO
Correct answer is option 'A'. Can you explain this answer?
Which is the reducing agent in the reaction ?
ZnO + C → Zn + CO
a)
C
b)
Zn
c)
CO
d)
ZnO
|
|
Amit Sharma answered |
C gets oxidised to CO and hence is a reducing agent.
Which one of the following four metals would be displaced from the solution of its salt by other three metals ?- a)Mg
- b)Ag
- c)Zn
- d)Cu
Correct answer is option 'B'. Can you explain this answer?
Which one of the following four metals would be displaced from the solution of its salt by other three metals ?
a)
Mg
b)
Ag
c)
Zn
d)
Cu
|
|
Gaurav Kumar answered |
Silver because it is the least reactive among these metals.
The reaction Pb(NO3)2 + 2NaI → 2NaNO3 + PbI2 ↓ is classified as- a)Combination reaction
- b)Decomposition reaction
- c)Single replacement reaction
- d)Double replacement reaction
Correct answer is option 'D'. Can you explain this answer?
The reaction Pb(NO3)2 + 2NaI → 2NaNO3 + PbI2 ↓ is classified as
a)
Combination reaction
b)
Decomposition reaction
c)
Single replacement reaction
d)
Double replacement reaction
|
|
Pooja Shah answered |
In this reaction, two new substances lead iodide, and sodium nitrate are formed by an interchange of radicals in lead nitrate and sodium iodide.
Chapter doubts & questions for Chemical Reactions and Equations - Online MCQ Tests for Class 10 2025 is part of Class 10 exam preparation. The chapters have been prepared according to the Class 10 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 10 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Reactions and Equations - Online MCQ Tests for Class 10 in English & Hindi are available as part of Class 10 exam.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Related Class 10 Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









