Class 10 Exam > Class 10 Questions > Which of the following metals comes above zin...
Start Learning for Free
Which of the following metals comes above zinc in reactivity series?
- a)Silver
- b)Copper
- c)Aluminium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which of the following metals comes above zinc in reactivity series?a)...
Reactivity Series:
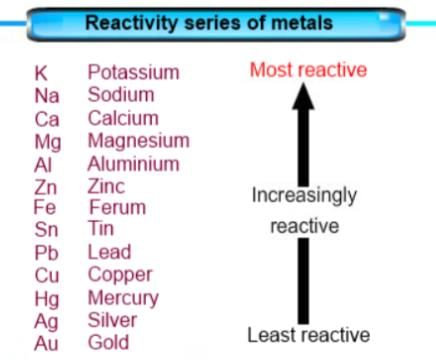
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
View all questions of this test
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
Most Upvoted Answer
Which of the following metals comes above zinc in reactivity series?a)...
The other metals are less reactive than zinc.

Free Test
FREE
| Start Free Test |
Community Answer
Which of the following metals comes above zinc in reactivity series?a)...
Yes it is aluminium you can learn it by this way: kedar -- K-- potassium Nath -- Na-- sodium Ca. -- Ca-- calcium Maali. ---Mg-- Magnesium Aalu. ---Al -- Aluminium Zankar ---Zn --- zinc Feke --Fe -- Iron Pakata. --Pb-- lead Hai -- H- hydrogen...,.,.,.,.,.,( Kedar Nath Ca Maali Aalu Zankar Feke Pakata Hai )

|
Explore Courses for Class 10 exam
|

|
Question Description
Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer?.
Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following metals comes above zinc in reactivity series?a)Silverb)Copperc)Aluminiumd)IronCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























