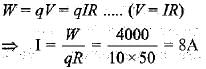All Exams >
Class 10 >
NTSE for Class 10 >
All Questions
All questions of Science for Class 10 Exam
Which of the following is a combination reaction?
- a)CaCO3 → CaO + CO2
- b)H2 + Cl2 → 2HCl
- c)H2CO3 → H2O + CO2
- d)2KClO3 → 2KCl + 3O2
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a combination reaction?
a)
CaCO3 → CaO + CO2
b)
H2 + Cl2 → 2HCl
c)
H2CO3 → H2O + CO2
d)
2KClO3 → 2KCl + 3O2
|
|
Roshni chauhan answered |
Balanced Equation:
H2 + Cl2 → 2HCl2
H2 + Cl2 → 2HCl2
The above reaction is an example of combination reaction because two different elements are combining to form a single compound.
Which of the following is a decomposition reaction?- a)NaOH + HCl → NaCl + H2O
- b)NH4CNO → H2NCONH2
- c)2KClO3 → 2KCl + 3O2
- d)H2 + I2 → 2HI
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a decomposition reaction?
a)
NaOH + HCl → NaCl + H2O
b)
NH4CNO → H2NCONH2
c)
2KClO3 → 2KCl + 3O2
d)
H2 + I2 → 2HI
|
|
Meera Rana answered |
A decomposition reaction occurs when one reactant breaks down into two or more products. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
2KClO3 → 2KCl + 3O2
2KClO3 → 2KCl + 3O2
Which of the following does not conduct electricity?- a)Sodium hydroxide
- b)Rain water
- c)Hydrochloric acid
- d)Distilled water
Correct answer is option 'D'. Can you explain this answer?
Which of the following does not conduct electricity?
a)
Sodium hydroxide
b)
Rain water
c)
Hydrochloric acid
d)
Distilled water
|
|
Gaurav Kumar answered |
Distilled water do not conduct electricity. The reason is that a liquid conducts electricity is by the positively or negatively charged ions that are actually moving from one of the electrodes to the other, carrying charge (electricity) with them.
The reaction C + O2 → CO2 + Heat is a:
- a)Combination reaction
- b)Oxidation reaction
- c)Exothermic reaction
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
The reaction C + O2 → CO2 + Heat is a:
a)
Combination reaction
b)
Oxidation reaction
c)
Exothermic reaction
d)
All of the above
|
|
Amit Sharma answered |
C + O2 → CO2 + Heat
- This is a Combination reaction because C and O2 are combining to produce one single compound CO2.
- This is also an Oxidation reaction because carbon is getting oxidized.
- This is also an exothermic reaction because in this reaction heat is getting released.
So Option D is correct
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?- a)Red litmus solution
- b)Lime water
- c)A burning splinter
- d)Blue litmus solution
Correct answer is option 'C'. Can you explain this answer?
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?
a)
Red litmus solution
b)
Lime water
c)
A burning splinter
d)
Blue litmus solution
|
|
Kiran Mehta answered |
A burning splinter since hydrogen gas burns with a pop sound.
Zinc being an active metal readily reacts with hydrochloric acid at room temperature to form soluble zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2 + H2.
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:- a)Decomposition reaction
- b)Combination reaction
- c)Displacement reaction
- d)Single displacement reaction
Correct answer is option 'C'. Can you explain this answer?
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
The above reaction is an example of a:
a)
Decomposition reaction
b)
Combination reaction
c)
Displacement reaction
d)
Single displacement reaction

|
Anushka Chopra answered |
The given equation is a displacement reaction in which Fe of Fe2O3 has been displaced by Al. Hence, (c) is the correct option.
How many elements were known when Mendeleev started his work on his periodic table?- a)63
- b)55
- c)69
- d)45
Correct answer is option 'A'. Can you explain this answer?
How many elements were known when Mendeleev started his work on his periodic table?
a)
63
b)
55
c)
69
d)
45

|
Deepesh Singh Sengar. answered |
There is 63 element because Mendel put element according to their mass volumes
upvote my answer
upvote my answer
To get 2Ω resistance using only 6Ω resistors, the number of them required is- a)2
- b)3
- c)4
- d)6
Correct answer is option 'B'. Can you explain this answer?
To get 2Ω resistance using only 6Ω resistors, the number of them required is
a)
2
b)
3
c)
4
d)
6
|
|
Avinash Patel answered |
Three resistors of 2Ω is required to get 6Ω because resultant is more than individual so they all must be connected in series.
Can you explain the answer of this question below:ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
- A:
A = 1, B = 1, C = 2, D = 1
- B:
A = 2, B = 2, C = 2, D = 1
- C:
A = 2, B = 1, C = 2, D = 1
- D:
none of these
The answer is b.
ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
A = 1, B = 1, C = 2, D = 1
A = 2, B = 2, C = 2, D = 1
A = 2, B = 1, C = 2, D = 1
none of these
|
|
Shweta singh answered |
2 Na + 2H2O gives 2NaOH + H2
So option B is Correct answer.
The best conductor of electricity among the following is:- a)Hg (Mercury)
- b)Al (Aluminum)
- c)Ag (Silver)
- d)Au (Gold)
Correct answer is option 'C'. Can you explain this answer?
The best conductor of electricity among the following is:
a)
Hg (Mercury)
b)
Al (Aluminum)
c)
Ag (Silver)
d)
Au (Gold)
|
|
Avinash Patel answered |
Metals are good conductors of electricity. silver is found in in pure form from the earth. so In the given options all are metals. Among them silver is the best conductor of electricity after second best conductor of electricity is gold.
Metal which is a poor conductor of heat is:- a)Zinc
- b)Lead
- c)Gold
- d)Copper
Correct answer is option 'B'. Can you explain this answer?
Metal which is a poor conductor of heat is:
a)
Zinc
b)
Lead
c)
Gold
d)
Copper
|
|
Anjana Khatri answered |
Although metals are supposed to be good conductors of electricity and heat,metals like mercury, lead, alloys of iron and chromium, titanium and stainless steel are poor conductors when compared to silver, copper and gold.
The effective resistance between A and B is
- a)4Ω
- b)6Ω
- c)May be 10Ω
- d)Must be 10Ω
Correct answer is option 'A'. Can you explain this answer?
The effective resistance between A and B is


a)
4Ω
b)
6Ω
c)
May be 10Ω
d)
Must be 10Ω
|
|
Gaurav Kumar answered |
6Ω is shorted
as current will pass from wire having no resistance
so effective resistance is 4Ω.
as current will pass from wire having no resistance
so effective resistance is 4Ω.
Which of the following is a displacement reaction?- a)CaCO3 → CaO + CO2
- b)CaO + 2HCl → CaCl2 + H2O
- c)Fe + CuSO4 → FeSO4 + Cu
- d)NaOH + HCl → NaCl + H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a displacement reaction?
a)
CaCO3 → CaO + CO2
b)
CaO + 2HCl → CaCl2 + H2O
c)
Fe + CuSO4 → FeSO4 + Cu
d)
NaOH + HCl → NaCl + H2O
|
|
Gaurav Kumar answered |
Fe + CuSo4 → FeSO4 + Cu
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
(a) is a decomposition reaction.
(b) and (d) are neutralisation reactions.
(b) and (d) are neutralisation reactions.
Calculate the current flows through the 10Ω resistor in the following circuit.
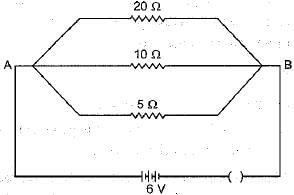
- a)1.2A
- b)0.6A
- c)0.2A
- d)2.0A
Correct answer is option 'B'. Can you explain this answer?
Calculate the current flows through the 10Ω resistor in the following circuit.


a)
1.2A
b)
0.6A
c)
0.2A
d)
2.0A

|
Sun Ray Institute answered |
In parallel, potential difference across each resistor will remain same. So, current through 10Ω resistor
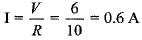
Which of the following is an example of displacement reaction?- a)4 Na+ O2 → 2 Na2O
- b)2 Cu + O2 → 2 CuO
- c)Mg + 2 HCl → MgCl2 + H2
- d)N2 + 3 H2 → 2 NH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of displacement reaction?
a)
4 Na+ O2 → 2 Na2O
b)
2 Cu + O2 → 2 CuO
c)
Mg + 2 HCl → MgCl2 + H2
d)
N2 + 3 H2 → 2 NH3

|
Orion Classes answered |
It is a single replacement reaction.
Mg + 2HCl → MgCl2 + H2
Mg + 2HCl → MgCl2 + H2
Identify the amphoteric oxide from the following:- a)MgO
- b)Na2O
- c)Al2O3
- d)K2O
Correct answer is option 'C'. Can you explain this answer?
Identify the amphoteric oxide from the following:
a)
MgO
b)
Na2O
c)
Al2O3
d)
K2O
|
|
Arun Sharma answered |
Amphoteric metal oxides react with both acids as well as bases to produce salt and water.
Aluminium oxide is an amphoteric oxide because it reacts with acids as well as bases and form salts and water.
Al2O3 + 6HCl → 2AlCl3 + 3H2O (Basic nature)
Aluminium Hydrochloric Aluminium Water
oxide acid chloride
Al2O3 + 2NaOH → 2NaAlO2 + H2O (Acidic nature)
Aluminium Sodium Sodium Water
oxide Hydroxide aluminate
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O- a)CuCl
- b)Cu(OH)2
- c)CuCl2
- d)HOCl
Correct answer is option 'C'. Can you explain this answer?
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O
a)
CuCl
b)
Cu(OH)2
c)
CuCl2
d)
HOCl
|
|
Vikram Kapoor answered |
When copper oxide and dilute hydrochloric acid are mixed the blue green solution is formed.
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
Which of the following metals comes above zinc in reactivity series?
- a)Silver
- b)Copper
- c)Aluminium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals comes above zinc in reactivity series?
a)
Silver
b)
Copper
c)
Aluminium
d)
Iron
|
|
Karthik murthy answered |
Reactivity Series:
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
All metals exist as solid at room temperature except the metal ‘X’. Name the metal “X’.- a)Tungsten
- b)Mercury
- c)Gold
- d)Potassium
Correct answer is option 'B'. Can you explain this answer?
All metals exist as solid at room temperature except the metal ‘X’. Name the metal “X’.
a)
Tungsten
b)
Mercury
c)
Gold
d)
Potassium
|
|
Ananya Das answered |
It is the only metal that is liquid at room temperature. It has the lowest melting point and boiling point of any other metal. It has low thermal conductivity, and a quite low electrical conductivity. It is the only metal that doesn’t form diatomic molecules in the gaseous phase.
Depletion of ozone is mainly due to- a)chlorofluorocarbon compounds
- b)carbon monoxide
- c)methane
- d)pesticides
Correct answer is option 'A'. Can you explain this answer?
Depletion of ozone is mainly due to
a)
chlorofluorocarbon compounds
b)
carbon monoxide
c)
methane
d)
pesticides
|
|
Rahul tiwari answered |
Depletion of ozone layer is mainly due to chlorofluorocarbon compounds.
Which metal is present in Calcium Hydroxide?- a)C
- b)O
- c)Ca
- d)H
Correct answer is option 'C'. Can you explain this answer?
Which metal is present in Calcium Hydroxide?
a)
C
b)
O
c)
Ca
d)
H
|
|
Amit Sharma answered |
Calcium hydroxide Ca ( OH) 2 , has calcium ( Ca) which is a metal.
The colour of phenolphthalein in acids is:- a)Colourless
- b)Red
- c)Pink
- d)Blue
Correct answer is option 'A'. Can you explain this answer?
The colour of phenolphthalein in acids is:
a)
Colourless
b)
Red
c)
Pink
d)
Blue
|
|
Krishna Iyer answered |
Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colourless in acidic solutions and magenta in basic solutions.
The magnetic field lines inside a long current-carrying solenoid are near-
- a)Straight
- b)Circular
- c)Elliptical
- d)Parabolic
Correct answer is option 'A'. Can you explain this answer?
The magnetic field lines inside a long current-carrying solenoid are near-
a)
Straight
b)
Circular
c)
Elliptical
d)
Parabolic

|
Gunjan Lakhani answered |
The field lines inside the solenoid are in the form of parallel straight lines. This indicates that the magnetic field is the same at all points inside the solenoid. That is, the field is uniform inside the solenoid.
Carbon dioxide is an example of:- a)Amphoteric oxide
- b)Acidic oxide
- c)Basic oxide
- d)Neutral oxide
Correct answer is option 'B'. Can you explain this answer?
Carbon dioxide is an example of:
a)
Amphoteric oxide
b)
Acidic oxide
c)
Basic oxide
d)
Neutral oxide
|
|
Gaurav Kumar answered |
Acid oxides is a complex chemical substance oxides, which form a salt with the chemical reactions with bases or basic oxides and do not react with acidic oxides.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
The reaction H2 + Cl2 → 2HCl is a –- a)Decomposition reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Displacement reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction H2 + Cl2 → 2HCl is a –
a)
Decomposition reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Displacement reaction
|
|
Anjana Khatri answered |
It is a reaction in which two substances react with each other to make a single substance. Therefore, H2 + CL2 = 2HCL is an COMBINATION REACTION. It is a special case of addition reaction known as synthesis.
Transparent medium is one :
- a)Which allows light to pass through
- b)Which absorbs most of the light
- c)Which do not allows light to pass through
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Transparent medium is one :
a)
Which allows light to pass through
b)
Which absorbs most of the light
c)
Which do not allows light to pass through
d)
None of these

|
Imk Pathshala answered |
A transparent medium is a material that partially allows light to pass through.
When light encounters a transparent medium, it can penetrate the material and transmit through it.
Examples of transparent mediums include glass, water, and air.
Transparent mediums are essential for various applications such as optics, windows, and lenses.
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
- a)SO2
- b)H2S
- c)H2O
- d)S
Correct answer is option 'B'. Can you explain this answer?
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
a)
SO2
b)
H2S
c)
H2O
d)
S
|
|
Raghav Bansal answered |
- In the reaction SO2 is getting reduced to S as oxygen is being removed.
- Similarly, H2S is oxidised to S as hydrogen is removed.
- The species getting oxidised facilitates the reduction process and is the reducing agent.
- Hence, H2S is the reducing agent and Oxidising agent is SO2.
So, option B is correct
The Law of Octaves was applicable only upto element ________.- a)Sodium
- b)Calcium
- c)Zinc
- d)Copper
Correct answer is option 'B'. Can you explain this answer?
The Law of Octaves was applicable only upto element ________.
a)
Sodium
b)
Calcium
c)
Zinc
d)
Copper
|
|
Vikram Kapoor answered |
The major limitations of Newlands' law of octaves were :
It was applicable to only lighter elements having atomic masses upto 40 u, i.e., upto calcium. After calcium, the first and the eighth element did not have similar properties. For example chromium (Cr) and yttrium (Y) are the first and the eighth element placed in the same column but they have entirely different properties.
It was applicable to only lighter elements having atomic masses upto 40 u, i.e., upto calcium. After calcium, the first and the eighth element did not have similar properties. For example chromium (Cr) and yttrium (Y) are the first and the eighth element placed in the same column but they have entirely different properties.
Magnetic lines of force originate from the- a)North pole
- b)Center point
- c)South pole
- d)Either north pole or south pole
Correct answer is option 'A'. Can you explain this answer?
Magnetic lines of force originate from the
a)
North pole
b)
Center point
c)
South pole
d)
Either north pole or south pole
|
|
Ananya Das answered |
The direction of magnetic line of force is the direction of force on a North Pole, so the magnetic lines of force always begin on the North Pole of a magnet and end on the South Pole of the magnet. When a small magnetic compass is placed along a lie of force, it sets itself along the line tangential to it. Hence, the line drawn from the South Pole of the compass to its North pole shows the direction of the magnetic field.
Which of the following non-metal is good conductor of electricity?- a)Graphite
- b)Phosphorus
- c)Hydrogen
- d)Bromine
Correct answer is option 'A'. Can you explain this answer?
Which of the following non-metal is good conductor of electricity?
a)
Graphite
b)
Phosphorus
c)
Hydrogen
d)
Bromine
|
|
Gaurav Kumar answered |
Carbon, in the form of Graphite is a good conductor of electricity. It conducts heat and electricity like a metal or a metalloid.
Can you explain the answer of this question below:Haemoglobin is –
- A:
Vitamin
- B:
Skin pigment
- C:
Blood carrier
- D:
Respiratory pigment
The answer is D.
Haemoglobin is –
Vitamin
Skin pigment
Blood carrier
Respiratory pigment
|
|
Krishna Iyer answered |
the hemoglobin increases the oxygen carrying capacity of blood. In humans and most other vertebrates, the most common respiratory pigment is a protein called hemoglobin.
Asexual reproduction is good because it:- a)Involves two parents
- b)Is simple and fast
- c)Shows no variation
- d)Produces defect in offspring
Correct answer is option 'B'. Can you explain this answer?
Asexual reproduction is good because it:
a)
Involves two parents
b)
Is simple and fast
c)
Shows no variation
d)
Produces defect in offspring
|
|
Anjana Khatri answered |
Asexual reproduction occurs when an organism makes more of itself without exchanging genetic information with another organism through sex.In sexually reproducing organisms, the genomes of two parents are combined to create offspring with unique genetic profiles. This is beneficial to the population because genetically diverse populations have a higher chance of withstanding survival challenges such as disease and environmental changes.
Asexually reproducing organisms can suffer a dangerous lack of diversity – but they can also reproduce faster than sexually reproducing organisms, and a single individual can found a new population without the need for a mate.
An electric current flowing through a conductor produces
- a)Magnetic field
- b)Electric Field
- c)Electromagnetic field
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
An electric current flowing through a conductor produces
a)
Magnetic field
b)
Electric Field
c)
Electromagnetic field
d)
None of these
|
|
Vikas Kumar answered |
An electric current through a metallic conductor produces a magnetic field around it.
Which of the following represent a double displacement reaction?- a)2H2 + O2 → 2H2O
- b)2Mg + O2 → 2MgO
- c)AgNO3 + NaCl → AgCl
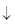 + NaNO3
+ NaNO3 - d)H2 + Cl2 → 2HCl
Correct answer is option 'C'. Can you explain this answer?
Which of the following represent a double displacement reaction?
a)
2H2 + O2 → 2H2O
b)
2Mg + O2 → 2MgO
c)
AgNO3 + NaCl → AgCl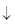 + NaNO3
+ NaNO3
d)
H2 + Cl2 → 2HCl

|
Environment Lover answered |
Yah the correct answer is c as it follow the definition of displacement reaction
Identify the type of reaction: HCl + NaOH → NaCl + H2O- a)Combination reaction
- b)Double decomposition reaction
- c)Decomposition reaction
- d)Neutralisation reaction
Correct answer is option 'D'. Can you explain this answer?
Identify the type of reaction: HCl + NaOH → NaCl + H2O
a)
Combination reaction
b)
Double decomposition reaction
c)
Decomposition reaction
d)
Neutralisation reaction
|
|
Krishna Iyer answered |
Reaction of a strong acid with strong base is called neutralization reaction which produces salt and water,
HCl + NaOH → NaCl + H2O
This equation is already balanced.
What happens when non-metals react with water?a) Hydrogen gas is formedb) Carbon dioxide gas is formedc) Non-metals do not react with waterd) None of these.Correct answer is option 'C'. Can you explain this answer?
b) Carbon dioxide gas is formed
c) Non-metals do not react with water
d) None of these.
Correct answer is option 'C'. Can you explain this answer?
|
|
Pooja Shah answered |
Non - metals do not react with water to evolve hydrogen gas. ( no reaction)
Ability of the eye to see objects at all distances... more is calleda)Binocular visionb)Myopiac)Accommodationd)HypermetropiaCorrect answer is option 'C'. Can you explain this answer?
|
|
Ishan Choudhury answered |
Changes in the contraction of the ciliary muscles alter the focal distance of the eye, causing nearer or future images to come into focus on the retina; this process is known as accommodation.
Which part of seed on germination develops into shoot?- a)Funicle
- b)Cotyledon
- c)Radicle
- d)Plumule
Correct answer is option 'D'. Can you explain this answer?
Which part of seed on germination develops into shoot?
a)
Funicle
b)
Cotyledon
c)
Radicle
d)
Plumule
|
|
Rahul Kapoor answered |
In plant physiology, the epicotyl is the embryonic shoot above the cotyledons. In most plants theepicotyl will eventually develop into the leaves of the plant. In dicots, the hypocotyl is what appears to be the base stem under the spent withered cotyledons, and the shoot just above that is theepicotyl.
Which is commonly called as plumule , which forms shoot parts And radicle forms root parts.
Which one is more metallic element ?- a)Na
- b)Mg
- c)Al
- d)Si
Correct answer is option 'A'. Can you explain this answer?
Which one is more metallic element ?
a)
Na
b)
Mg
c)
Al
d)
Si
|
|
Diya Sharma answered |
Because it more fastly or quickly loss it's electron.
If four resistors each of 1Ω are connected in parallel, the effective resistance will be- a)0.5Ω
- b)0.25Ω
- c)4Ω
- d)2Ω
Correct answer is option 'B'. Can you explain this answer?
If four resistors each of 1Ω are connected in parallel, the effective resistance will be
a)
0.5Ω
b)
0.25Ω
c)
4Ω
d)
2Ω
|
|
Gaurav Kumar answered |
If four resistors each of 1Ω are connected in parallel, the effective resistance will be 0.25Ω..
As, in parralel connection,
1/Reff. = 1/R1 + 1/R2 + ....
A lustrous non-metal is:- a)Iodine
- b)Bromine
- c)Chlorine
- d)Sulphur
Correct answer is option 'A'. Can you explain this answer?
A lustrous non-metal is:
a)
Iodine
b)
Bromine
c)
Chlorine
d)
Sulphur
|
|
Pooja Shah answered |
Non - metals do not have luster. They do not reflect light from their surface ( except iodine and diamond) . So, here a lustrous now Metal is iodine.
The human eye forms the image of an object at its- a)Iris
- b)Pupil
- c)Cornea
- d)Retina
Correct answer is option 'D'. Can you explain this answer?
The human eye forms the image of an object at its
a)
Iris
b)
Pupil
c)
Cornea
d)
Retina
|
|
Rahul Kapoor answered |
Retina is the inner back portion of eye. Retina is composed of enormous number of light sensitive cells. Lens forms image of the visual world on retina through cornea. Light sensitive cells of retina get activated upon illumination and generate electric signals. Optic nerves send these electric signals to the brain, where these signals finally processed and interpreted to see an object as they are.
Which one of the following metal reacts vigorously with oxygen and water?
- a)Sodium
- b)Iron
- c)Calcium
- d)Magnesium
Correct answer is option 'A'. Can you explain this answer?
Which one of the following metal reacts vigorously with oxygen and water?
a)
Sodium
b)
Iron
c)
Calcium
d)
Magnesium
|
|
Ananya Das answered |
Sodium metal reacts vigorously with oxygen and water.
The lustre of a metal is due to:- a)Its high density
- b)Presence of free electrons
- c)Its high polishing
- d)Its chemical inertness
Correct answer is option 'B'. Can you explain this answer?
The lustre of a metal is due to:
a)
Its high density
b)
Presence of free electrons
c)
Its high polishing
d)
Its chemical inertness

|
Rohini Seth answered |
The lustre of a metal is due to presence of free electrons.
It has to do with the way light and electrons on the surface of metals interact. The outer electrons in a metal are almost not bound to any individual atom, thus are relatively free, and are concentrated on the surface. These electrons (electron density) tend to oscillate at a collective frequency.
The colour of zinc metal is
- a)reddish-brown
- b)grey
- c)Blue
- d)silvery-white
Correct answer is option 'D'. Can you explain this answer?
The colour of zinc metal is
a)
reddish-brown
b)
grey
c)
Blue
d)
silvery-white
|
|
Vikas Kumar answered |
The correct option is D
silvery-white
Explanation for correct option
(D) The color of Zinc metal is silvery.
- An electron gets excited to a higher energy orbital from a lower energy d orbital. The frequency of light absorbed is proportional to the excitation energy.
- This frequency is usually in the visible range.
- The color seen corresponds to the complementary color of the light absorbed.
- Zinc (Zn) is a silvery-white metal.
Hence, option (D) is correct. The color of Zinc metal is silvery.
Which of the following has the maximum non-metallic character ?- a)F
- b)Cl
- c)Br
- d)I
Correct answer is option 'A'. Can you explain this answer?
Which of the following has the maximum non-metallic character ?
a)
F
b)
Cl
c)
Br
d)
I
|
|
Ananya Das answered |
Non metallic character decreases as we move down the group. In a group, the size of an element increases because there is an addition of new shell and electron is added in that shell. Hence, fluorine has the most non metallic character.
Which of the following is not an example of chemical change?- a)rusting of iron
- b)milk changes to curd
- c)digestion of food in our body
- d)changing of water to water vapour
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an example of chemical change?
a)
rusting of iron
b)
milk changes to curd
c)
digestion of food in our body
d)
changing of water to water vapour
|
|
Amit Sharma answered |
The process of changing water vapour into water is called condensation. It is not a chemical reaction.
When milk converts in curd the process is irreversible and a new substance with different properties is formed hence it is an example of a chemical change.
Rusting of iron is also a chemical change.
Chapter doubts & questions for Science - NTSE for Class 10 2025 is part of Class 10 exam preparation. The chapters have been prepared according to the Class 10 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 10 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Science - NTSE for Class 10 in English & Hindi are available as part of Class 10 exam.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup