All Exams >
RRB Group D / RPF Constable >
General Science for Competitive Exams >
All Questions
All questions of Class 10 Chemistry for RRB Group D / RPF Constable Exam
Which of the following is a combination reaction?
- a)CaCO3 → CaO + CO2
- b)H2 + Cl2 → 2HCl
- c)H2CO3 → H2O + CO2
- d)2KClO3 → 2KCl + 3O2
Correct answer is option 'B'. Can you explain this answer?
Which of the following is a combination reaction?
a)
CaCO3 → CaO + CO2
b)
H2 + Cl2 → 2HCl
c)
H2CO3 → H2O + CO2
d)
2KClO3 → 2KCl + 3O2
|
|
Roshni chauhan answered |
Balanced Equation:
H2 + Cl2 → 2HCl2
H2 + Cl2 → 2HCl2
The above reaction is an example of combination reaction because two different elements are combining to form a single compound.
Which of the following is a decomposition reaction?- a)NaOH + HCl → NaCl + H2O
- b)NH4CNO → H2NCONH2
- c)2KClO3 → 2KCl + 3O2
- d)H2 + I2 → 2HI
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a decomposition reaction?
a)
NaOH + HCl → NaCl + H2O
b)
NH4CNO → H2NCONH2
c)
2KClO3 → 2KCl + 3O2
d)
H2 + I2 → 2HI
|
|
Meera Rana answered |
A decomposition reaction occurs when one reactant breaks down into two or more products. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
2KClO3 → 2KCl + 3O2
2KClO3 → 2KCl + 3O2
The reaction C + O2 → CO2 + Heat is a:
- a)Combination reaction
- b)Oxidation reaction
- c)Exothermic reaction
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
The reaction C + O2 → CO2 + Heat is a:
a)
Combination reaction
b)
Oxidation reaction
c)
Exothermic reaction
d)
All of the above
|
|
Amit Sharma answered |
C + O2 → CO2 + Heat
- This is a Combination reaction because C and O2 are combining to produce one single compound CO2.
- This is also an Oxidation reaction because carbon is getting oxidized.
- This is also an exothermic reaction because in this reaction heat is getting released.
So Option D is correct
Metal have ............no. of electrons in their outer most shell –
a)1 to 8b)7 to 9c)1 to 3d)10 to 12Correct answer is option 'C'. Can you explain this answer?
|
|
Gaurav Kumar answered |
Metals have 1-3 electrons in their outermost orbit. They are present on the extreme left of the periodic table. They tend to donate electrons and obtain the stable electronic configuration of the nearest noble gas. The valency on metals also ranges from 1-3 .
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?- a)Red litmus solution
- b)Lime water
- c)A burning splinter
- d)Blue litmus solution
Correct answer is option 'C'. Can you explain this answer?
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?
a)
Red litmus solution
b)
Lime water
c)
A burning splinter
d)
Blue litmus solution
|
|
Kiran Mehta answered |
A burning splinter since hydrogen gas burns with a pop sound.
Zinc being an active metal readily reacts with hydrochloric acid at room temperature to form soluble zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2 + H2.
The major constituent of biogas is- a)Propane
- b)Acetylene
- c)Methane
- d)Benzene
Correct answer is option 'C'. Can you explain this answer?
The major constituent of biogas is
a)
Propane
b)
Acetylene
c)
Methane
d)
Benzene
|
|
Vikram Kapoor answered |
Biogas comprises primarily methane (CH4) and carbon dioxide (CO2) and may have small amounts of hydrogen sulphide (H2S), moisture and siloxanes.
Can you explain the answer of this question below:ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
- A:
A = 1, B = 1, C = 2, D = 1
- B:
A = 2, B = 2, C = 2, D = 1
- C:
A = 2, B = 1, C = 2, D = 1
- D:
none of these
The answer is b.
ANa+BH2O→CNaOH+DH2 . What would come in place of A , B C and D to balance the equation
A = 1, B = 1, C = 2, D = 1
A = 2, B = 2, C = 2, D = 1
A = 2, B = 1, C = 2, D = 1
none of these
|
|
Shweta singh answered |
2 Na + 2H2O gives 2NaOH + H2
So option B is Correct answer.
The reactions in which more reactive element can displace less reactive element from a compound are called- a)double displacement reaction
- b)Displacement reaction
- c)Decomposition
- d)combination reaction
Correct answer is option 'B'. Can you explain this answer?
The reactions in which more reactive element can displace less reactive element from a compound are called
a)
double displacement reaction
b)
Displacement reaction
c)
Decomposition
d)
combination reaction
|
|
Krishna Iyer answered |
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Both metals and non-metals take part in displacement reactions.
Which of the following is a displacement reaction?- a)CaCO3 → CaO + CO2
- b)CaO + 2HCl → CaCl2 + H2O
- c)Fe + CuSO4 → FeSO4 + Cu
- d)NaOH + HCl → NaCl + H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a displacement reaction?
a)
CaCO3 → CaO + CO2
b)
CaO + 2HCl → CaCl2 + H2O
c)
Fe + CuSO4 → FeSO4 + Cu
d)
NaOH + HCl → NaCl + H2O
|
|
Gaurav Kumar answered |
Fe + CuSo4 → FeSO4 + Cu
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
is displacement reaction.
In these reaction iron (Fe) displace copper (Cu)
Hence, it is a displacement type of reaction.
(a) is a decomposition reaction.
(b) and (d) are neutralisation reactions.
(b) and (d) are neutralisation reactions.
Which one of Fe, Al, Cu, Zn is the least reactive metal?- a)Cu
- b)Al
- c)Fe
- d)Zn
Correct answer is option 'A'. Can you explain this answer?
Which one of Fe, Al, Cu, Zn is the least reactive metal?
a)
Cu
b)
Al
c)
Fe
d)
Zn

|
Jhimica Malik answered |
Least reactive metal is copper.
What is the nature of non metallic oxide- a)Amphoteric
- b)basic oxide
- c)Neutral oxide
- d)acidic oxide
Correct answer is option 'D'. Can you explain this answer?
What is the nature of non metallic oxide
a)
Amphoteric
b)
basic oxide
c)
Neutral oxide
d)
acidic oxide
|
|
Anjana Khatri answered |
Non-metals react with oxygen in the air to produce non-metal oxides. Here are two examples for the non-metals carbon and sulphur. Non-metal oxides such as sulphur dioxide and nitrogen oxide are responsible for acid rain. They dissolve in the water in the clouds to form acidic solutions.
10 ml of a solution of NaOH is found to be completely neutralised by 8mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl solution (the same solution as before) required to neutralise it will be–- a)4 mL
- b)8 mL
- c)12 mL
- d)16 mL
Correct answer is option 'D'. Can you explain this answer?
10 ml of a solution of NaOH is found to be completely neutralised by 8mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl solution (the same solution as before) required to neutralise it will be–
a)
4 mL
b)
8 mL
c)
12 mL
d)
16 mL
|
|
Rahul Kapoor answered |
Since 10 mL of NaOH solution requires 8 mL of HCl solution
Therefore, 20 mL of NaOH solution will require 8 * 2 = 16 mL of HCl solution
Which gas is liberated when HCl is added to a sample of solid Na2CO3 ?- a)Nitride
- b)carbide
- c)carbon mono oxide
- d)carbon dioxide
Correct answer is option 'D'. Can you explain this answer?
Which gas is liberated when HCl is added to a sample of solid Na2CO3 ?
a)
Nitride
b)
carbide
c)
carbon mono oxide
d)
carbon dioxide
|
|
Ishan Choudhury answered |
On heating it forms sodium carbonate (Na2CO3), B and CO2 gas, C is evolved. When CO2 gas is passed through lime water it forms calcium carbonate (CaCO3), which is slightly soluble in water making it milky.
Which metal is present in Calcium Hydroxide?- a)C
- b)O
- c)Ca
- d)H
Correct answer is option 'C'. Can you explain this answer?
Which metal is present in Calcium Hydroxide?
a)
C
b)
O
c)
Ca
d)
H
|
|
Amit Sharma answered |
Calcium hydroxide Ca ( OH) 2 , has calcium ( Ca) which is a metal.
The reaction H2 + Cl2 → 2HCl is a –- a)Decomposition reaction
- b)Combination reaction
- c)Double displacement reaction
- d)Displacement reaction
Correct answer is option 'B'. Can you explain this answer?
The reaction H2 + Cl2 → 2HCl is a –
a)
Decomposition reaction
b)
Combination reaction
c)
Double displacement reaction
d)
Displacement reaction
|
|
Anjana Khatri answered |
It is a reaction in which two substances react with each other to make a single substance. Therefore, H2 + CL2 = 2HCL is an COMBINATION REACTION. It is a special case of addition reaction known as synthesis.
Match the following with correct response.
(1) Gain of hydrogen
(2) Loss of hydrogen
(3) Unpleasant taste and foul small in fatty food
(4) Eating away of metal in the presence of air and moisture
(A) Reduction
(B) Rancidity
(C) Oxidation
(D) Corrosion- a)1-B, 2-D, 3-A, 4-C
- b)1-C, 2-B, 3-D, 4-A
- c)1-A, 2-C, 3-B, 4-D
- d)1-D, 2-A, 3-C, 4-B
Correct answer is option 'C'. Can you explain this answer?
Match the following with correct response.
(1) Gain of hydrogen
(2) Loss of hydrogen
(3) Unpleasant taste and foul small in fatty food
(4) Eating away of metal in the presence of air and moisture
(A) Reduction
(B) Rancidity
(C) Oxidation
(D) Corrosion
(1) Gain of hydrogen
(2) Loss of hydrogen
(3) Unpleasant taste and foul small in fatty food
(4) Eating away of metal in the presence of air and moisture
(A) Reduction
(B) Rancidity
(C) Oxidation
(D) Corrosion
a)
1-B, 2-D, 3-A, 4-C
b)
1-C, 2-B, 3-D, 4-A
c)
1-A, 2-C, 3-B, 4-D
d)
1-D, 2-A, 3-C, 4-B
|
|
Arun Sharma answered |
Reduction is the gain of hydrogen.. Oxidation is the loss of hydrogen.
Rancidity is the unpleasant taste and foul smell in fatty food.
Corrosion is eating away of metal in the presence of air and moisture.
Rancidity is the unpleasant taste and foul smell in fatty food.
Corrosion is eating away of metal in the presence of air and moisture.
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
- a)SO2
- b)H2S
- c)H2O
- d)S
Correct answer is option 'B'. Can you explain this answer?
In reaction SO2 + 2H2S → 2H2O + 3S, the reducing agent is
a)
SO2
b)
H2S
c)
H2O
d)
S
|
|
Raghav Bansal answered |
- In the reaction SO2 is getting reduced to S as oxygen is being removed.
- Similarly, H2S is oxidised to S as hydrogen is removed.
- The species getting oxidised facilitates the reduction process and is the reducing agent.
- Hence, H2S is the reducing agent and Oxidising agent is SO2.
So, option B is correct
Colour of copper sulphate solution changes when an iron nail is dipped in it because of- a)Decomposition reaction
- b)Displacement reaction
- c)Oxidation reaction
- d)Combination reaction
Correct answer is option 'B'. Can you explain this answer?
Colour of copper sulphate solution changes when an iron nail is dipped in it because of
a)
Decomposition reaction
b)
Displacement reaction
c)
Oxidation reaction
d)
Combination reaction
|
|
Ananya Das answered |
When an iron nail dipped in the copper sulphate solution than iron displaces copper from the copper sulphate because iron is more reactive than copper. Therefore the colour of the copper sulphate solution changes.
The reaction involved is
The reaction involved is
Which of the following non-metal is good conductor of electricity?- a)Graphite
- b)Phosphorus
- c)Hydrogen
- d)Bromine
Correct answer is option 'A'. Can you explain this answer?
Which of the following non-metal is good conductor of electricity?
a)
Graphite
b)
Phosphorus
c)
Hydrogen
d)
Bromine
|
|
Gaurav Kumar answered |
Carbon, in the form of Graphite is a good conductor of electricity. It conducts heat and electricity like a metal or a metalloid.
Which of the following represent a double displacement reaction?- a)2H2 + O2 → 2H2O
- b)2Mg + O2 → 2MgO
- c)AgNO3 + NaCl → AgCl
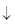 + NaNO3
+ NaNO3 - d)H2 + Cl2 → 2HCl
Correct answer is option 'C'. Can you explain this answer?
Which of the following represent a double displacement reaction?
a)
2H2 + O2 → 2H2O
b)
2Mg + O2 → 2MgO
c)
AgNO3 + NaCl → AgCl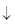 + NaNO3
+ NaNO3
d)
H2 + Cl2 → 2HCl

|
Environment Lover answered |
Yah the correct answer is c as it follow the definition of displacement reaction
P, Q, R are elements of Dobereiner’s triads. If the atomic mass of P is 7 and that of Q is 23, What will be the atomic mass of R?- a)15.0
- b)40.0
- c)30.0
- d)39.0
Correct answer is option 'D'. Can you explain this answer?
P, Q, R are elements of Dobereiner’s triads. If the atomic mass of P is 7 and that of Q is 23, What will be the atomic mass of R?
a)
15.0
b)
40.0
c)
30.0
d)
39.0
|
|
Gaurav Kumar answered |
In a Dobereiner's traid, the atomic mass of the middle element is roughly the average of the atomic mass of the other 2 elements. Thus,
Atomic mass of Q = (at. mass of P+ at. mass of R) /2
⇒ 23 = 7+ m(R) /2
⇒ 23*2 = 7+ m(R)
⇒ 46 -7 = m(R)
⇒ atomic mass of R = 39
Atomic mass of Q = (at. mass of P+ at. mass of R) /2
⇒ 23 = 7+ m(R) /2
⇒ 23*2 = 7+ m(R)
⇒ 46 -7 = m(R)
⇒ atomic mass of R = 39
Which is the least conductor of heat –- a)Gold
- b)Platinum
- c)Silver
- d)Lead
Correct answer is option 'D'. Can you explain this answer?
Which is the least conductor of heat –
a)
Gold
b)
Platinum
c)
Silver
d)
Lead
|
|
Abhavya Mishra answered |
Lead is an element having atomic no. 82.It is a poor conductor of heat because it easily reacts with the atmospheric oxygen and form lead oxide, and we know that metal oxide are poor conductor of heat and electricity.
The major constituent of natural gas is- a)Butane
- b)Methane
- c)Propane
- d)Ethane
Correct answer is option 'B'. Can you explain this answer?
The major constituent of natural gas is
a)
Butane
b)
Methane
c)
Propane
d)
Ethane
|
|
Amit Sharma answered |
Natural gas is primarily composed of methane, but also contains ethane, propane and heavier hydrocarbons. It also contains small amounts of nitrogen, carbon dioxide, hydrogen sulphide and trace amounts of water.
Which one is more metallic element ?- a)Na
- b)Mg
- c)Al
- d)Si
Correct answer is option 'A'. Can you explain this answer?
Which one is more metallic element ?
a)
Na
b)
Mg
c)
Al
d)
Si
|
|
Diya Sharma answered |
Because it more fastly or quickly loss it's electron.
Four solution P, Q, R, S have PH 2, 7, 9, 13 respectively. Which of the above solution will turn phenolphthalein pink?- a)S and R
- b)P only
- c)Q and S
- d)S only
Correct answer is option 'A'. Can you explain this answer?
Four solution P, Q, R, S have PH 2, 7, 9, 13 respectively. Which of the above solution will turn phenolphthalein pink?
a)
S and R
b)
P only
c)
Q and S
d)
S only
|
|
Ishan Choudhury answered |
Solution S and R will turn phenolphthalein pink because they are basic in nature and we know that bases turn phenolphthalein pink.
Which of the following salts contains water of crystallization.I. GypsumII. Epsum saltIII. Blue vitriolIV. Glauber’s salt- a)I and II
- b)III and IV
- c)I, II, III, IV
- d)II and IV
Correct answer is option 'C'. Can you explain this answer?
Which of the following salts contains water of crystallization.
I. Gypsum
II. Epsum salt
III. Blue vitriol
IV. Glauber’s salt
a)
I and II
b)
III and IV
c)
I, II, III, IV
d)
II and IV
|
|
Krishna Iyer answered |
Blue vitriol is copper sulphate Pentahydrate
CuSO4. 5H2O . It has 5 moles of water.
Gypsum is calcium sulphate dihydrate
CaSO4. 2H2O . It has two moles of water.
The chemical formula of Epsom salt is magnesium sulfate MgSO4(H2O) x, where x varies from 0 to 7.Glauber's salt is the Decahydrate form of sodium sulfate. NaSO4. 10H2O.
CuSO4. 5H2O . It has 5 moles of water.
Gypsum is calcium sulphate dihydrate
CaSO4. 2H2O . It has two moles of water.
The chemical formula of Epsom salt is magnesium sulfate MgSO4(H2O) x, where x varies from 0 to 7.Glauber's salt is the Decahydrate form of sodium sulfate. NaSO4. 10H2O.
Which one of the following metal reacts vigorously with oxygen and water?
- a)Sodium
- b)Iron
- c)Calcium
- d)Magnesium
Correct answer is option 'A'. Can you explain this answer?
Which one of the following metal reacts vigorously with oxygen and water?
a)
Sodium
b)
Iron
c)
Calcium
d)
Magnesium
|
|
Ananya Das answered |
Sodium metal reacts vigorously with oxygen and water.
Can you explain the answer of this question below:Mg ribbon burns with a dazzling flame in air (oxygen) and changes into a white substance, Magnesium oxide. Magnesium is
- A:
Neither oxidized nor reduced
- B:
Oxidized in the reaction
- C:
Reduced in the reaction
- D:
Both oxidized and reduced simultaneously
The answer is B.
Mg ribbon burns with a dazzling flame in air (oxygen) and changes into a white substance, Magnesium oxide. Magnesium is
Neither oxidized nor reduced
Oxidized in the reaction
Reduced in the reaction
Both oxidized and reduced simultaneously
|
|
Raghav Bansal answered |
When calcium oxide (chemical formula: Ca0) reacts with water (chemical formula: H20), the following reaction takes place:
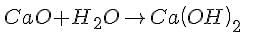
The product of this reaction is calcium hydroxide, also known as slaked lime. Thus, when calcium oxide reacts with water, slaked lime is produced.
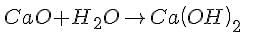
The product of this reaction is calcium hydroxide, also known as slaked lime. Thus, when calcium oxide reacts with water, slaked lime is produced.
The colour of zinc metal is
- a)reddish-brown
- b)grey
- c)Blue
- d)silvery-white
Correct answer is option 'D'. Can you explain this answer?
The colour of zinc metal is
a)
reddish-brown
b)
grey
c)
Blue
d)
silvery-white
|
|
Vikas Kumar answered |
The correct option is D
silvery-white
Explanation for correct option
(D) The color of Zinc metal is silvery.
- An electron gets excited to a higher energy orbital from a lower energy d orbital. The frequency of light absorbed is proportional to the excitation energy.
- This frequency is usually in the visible range.
- The color seen corresponds to the complementary color of the light absorbed.
- Zinc (Zn) is a silvery-white metal.
Hence, option (D) is correct. The color of Zinc metal is silvery.
How many elements are placed in lanthanide and actinide series?- a)57, 89
- b)14, 14
- c)89, 57
- d)14, 16
Correct answer is option 'B'. Can you explain this answer?
How many elements are placed in lanthanide and actinide series?
a)
57, 89
b)
14, 14
c)
89, 57
d)
14, 16
|
|
Neha Patel answered |
14 elements
The same holds for the actinide series that runs from atomic number 90 through to number 103, again 14 elements. Thus, as you move from thorium (Th) at element number 90, you begin to fill up the 5f sublevel and continue to fill up the 5f sublevel until you finish the actinide series at lawrencium (Lr).
Non-metal generally form –- a)Anions
- b)Cations
- c)Ions
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Non-metal generally form –
a)
Anions
b)
Cations
c)
Ions
d)
None of these
|
|
Rajiv Gupta answered |
Nonmetals tend to form anions. With one, two, or three valence electrons metals tend to lose electrons to achieve a noble gas configuration. (It would require more energy to gain the 5 – 7 electrons needed to fill their valence shell.)
Which of the following will give a pleasant smell of ester when heated with ethanol and a small quantity of sulphuric acid?- a)CH3COOH
- b)CH2CH2OH
- c)CH3OH
- d)CH3CHO
Correct answer is option 'A'. Can you explain this answer?
Which of the following will give a pleasant smell of ester when heated with ethanol and a small quantity of sulphuric acid?
a)
CH3COOH
b)
CH2CH2OH
c)
CH3OH
d)
CH3CHO
|
|
Arun Sharma answered |
Acetic acid gives a pleasant smell on rejection with ethanol due to the formation of ethyl acetate as :-
CH3COOH +C2H5OH → CH3COOC2H5 +H2O
CH3COOH +C2H5OH → CH3COOC2H5 +H2O
Which of the following has the maximum non-metallic character ?- a)F
- b)Cl
- c)Br
- d)I
Correct answer is option 'A'. Can you explain this answer?
Which of the following has the maximum non-metallic character ?
a)
F
b)
Cl
c)
Br
d)
I
|
|
Ananya Das answered |
Non metallic character decreases as we move down the group. In a group, the size of an element increases because there is an addition of new shell and electron is added in that shell. Hence, fluorine has the most non metallic character.
Which of the following is not an example of chemical change?- a)rusting of iron
- b)milk changes to curd
- c)digestion of food in our body
- d)changing of water to water vapour
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not an example of chemical change?
a)
rusting of iron
b)
milk changes to curd
c)
digestion of food in our body
d)
changing of water to water vapour
|
|
Amit Sharma answered |
The process of changing water vapour into water is called condensation. It is not a chemical reaction.
When milk converts in curd the process is irreversible and a new substance with different properties is formed hence it is an example of a chemical change.
Rusting of iron is also a chemical change.
A student added zinc granules to copper sulphate solution taken in a test tube. Out of the following, the correct observation (s) made by the student will be
I. zinc granules have no regular shape.
II. Zinc granules have silvery grey colour.
III. The colour of zinc granules changed to brownish black.- a)II only
- b)I, II and III
- c)III only
- d)I only
Correct answer is 'B'. Can you explain this answer?
A student added zinc granules to copper sulphate solution taken in a test tube. Out of the following, the correct observation (s) made by the student will be
I. zinc granules have no regular shape.
II. Zinc granules have silvery grey colour.
III. The colour of zinc granules changed to brownish black.
I. zinc granules have no regular shape.
II. Zinc granules have silvery grey colour.
III. The colour of zinc granules changed to brownish black.
a)
II only
b)
I, II and III
c)
III only
d)
I only
|
|
Shruti malhotra answered |
The displacement reaction that occurs is Zn(s)+ CuSO4(aq) → ZnSO4(aq)+Cu(s) (Brownish black)
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Carbon and its Compounds" of Class 10 Science, the questions are available for practiceQ. Which of the following will not decolourise bromine water?- a)C4H8
- b)C3H4
- c)C3H8
- d)C4H6
Correct answer is option 'C'. Can you explain this answer?
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Carbon and its Compounds" of Class 10 Science, the questions are available for practice
Q. Which of the following will not decolourise bromine water?
a)
C4H8
b)
C3H4
c)
C3H8
d)
C4H6
|
|
Krishna Iyer answered |
C3H8 is alkane and all other are alkene or alkyne. To decolourise the bromine water is the property of unsaturated compound not of saturated compound.
When conc acid is added to H2O, the reaction is- a)Exothermic
- b)Endothermic
- c)Neither EXO nor endothermic
- d)No reaction occur
Correct answer is option 'A'. Can you explain this answer?
When conc acid is added to H2O, the reaction is
a)
Exothermic
b)
Endothermic
c)
Neither EXO nor endothermic
d)
No reaction occur
|
|
Ananya Das answered |
When concentrated acid is added to water, the strong bonds of acid are broken and they dissociate into ions, thus releasing a large amount of energy in the form of heat.
Thus, the reaction is exothermic.
Thus, the reaction is exothermic.
Which of the following method is not used in preparing a base?- a)Burning of metal in air.
- b)Adding water to a metal oxide.
- c)Reaction between an acid and base.
- d)Heating metal carbonates.
Correct answer is option 'B'. Can you explain this answer?
Which of the following method is not used in preparing a base?
a)
Burning of metal in air.
b)
Adding water to a metal oxide.
c)
Reaction between an acid and base.
d)
Heating metal carbonates.
|
|
Pooja Shah answered |
The reaction between acid and base
It is a neutralization reaction which forms a salt but the base is not formed.
It is a neutralization reaction which forms a salt but the base is not formed.
According to Arrhenius acid gives –- a)H+ in water
- b)OH- in water
- c)Both (A) & (B)
- d)OH- in acid medium
Correct answer is option 'A'. Can you explain this answer?
According to Arrhenius acid gives –
a)
H+ in water
b)
OH- in water
c)
Both (A) & (B)
d)
OH- in acid medium
|
|
Vikram Kapoor answered |
According to Arrhenius, an acid. Is a substance which dissociates into H+ ion in water.
Which one of the following types of medicines is used for treating acidity?- a)Antacid
- b)Antibiotic
- c)Analgesic
- d)Antiseptic
Correct answer is option 'A'. Can you explain this answer?
Which one of the following types of medicines is used for treating acidity?
a)
Antacid
b)
Antibiotic
c)
Analgesic
d)
Antiseptic
|
|
Rahul Kapoor answered |
The indigestion is due to excess of acid produced in the stomach. The medicine used to neutralise it is called antacid
What happens when dilute HCl is added to iron fillings? Select the correct answer.- a)No reaction takes place
- b)chlorine gas and iron hydroxide are produced
- c)Iron salt and water are produced
- d)Hydrogen gas and iron chloride are produced
Correct answer is option 'D'. Can you explain this answer?
What happens when dilute HCl is added to iron fillings? Select the correct answer.
a)
No reaction takes place
b)
chlorine gas and iron hydroxide are produced
c)
Iron salt and water are produced
d)
Hydrogen gas and iron chloride are produced

|
Ashwani Mishra answered |
Hydrogen gas and iron chloride are produced.
Fe + HCl --> FeCl2 + H2
Fe + HCl --> FeCl2 + H2
The most malleable metal is –- a)Sodium
- b)Silium
- c)Gold
- d)Lead
Correct answer is option 'C'. Can you explain this answer?
The most malleable metal is –
a)
Sodium
b)
Silium
c)
Gold
d)
Lead
|
|
Neha Patel answered |
Gold is the most malleable of all metals; a single gram can be beaten into a sheet of 1 square meter, and an ounce into 300 square feet. Gold leaf can be beaten thin enough to become transparent." Pure Platinum is also quite plastic, as are pure Silver, pure copper, pure lead, and other metals.
Which of the following is not a strong acid?- a)H2SO4
- b)CH3COOH
- c)HNO3
- d)HCl
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not a strong acid?
a)
H2SO4
b)
CH3COOH
c)
HNO3
d)
HCl
|
|
Krishna Iyer answered |
Ch3COOH is weak acid as it dissociates only slightly in water.
Which of the following is an basic salt?- a)SnCl2
- b)NaCl
- c)NH4Cl
- d)CH3COONa
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an basic salt?
a)
SnCl2
b)
NaCl
c)
NH4Cl
d)
CH3COONa
|
|
Shivangi singhania answered |
Salt of weak acid and strong base: CH3COONa is a salt of weak acid CH3COOH and strong base NaOH. When it is dissolved in water it dissociates completely. ions of water get trapped and the pH of solution increases.
What happens when dil hydrochloric acid is added to iron fillings?- a)Hydrogen gas and Iron chloride are produced
- b)Chlorine gas and Iron hydroxide are produced
- c)NO reaction takes place
- d)Iron salt and water are produced
Correct answer is option 'A'. Can you explain this answer?
What happens when dil hydrochloric acid is added to iron fillings?
a)
Hydrogen gas and Iron chloride are produced
b)
Chlorine gas and Iron hydroxide are produced
c)
NO reaction takes place
d)
Iron salt and water are produced
|
|
Amit Sharma answered |
- Hydrogen gas and iron chloride are produced.
Fe + HCl → FeCl2 + H2
- The iron displaces hydrogen from hydrochloric acid to form iron (II) chloride & hydrogen gas. This is a single displacement reaction.
- Thus the answer is option (A) Hydrogen gas and iron chloride are produced.
What happens when copper metal is added to silver nitrate solution?- a)Cu will be reduced whereas silver will be oxidesed
- b)Copper being less reactive than Ag can’t displace silver from its solution
- c)Cu(NO3)2 will be formed
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
What happens when copper metal is added to silver nitrate solution?
a)
Cu will be reduced whereas silver will be oxidesed
b)
Copper being less reactive than Ag can’t displace silver from its solution
c)
Cu(NO3)2 will be formed
d)
None of these
|
|
Raghav Bansal answered |
The reaction is represented as follows:
2AgNO3 + Cu --------> Cu(NO3)2 + 2Ag ↓
Observation: Clear solution of AgNO3 turns Blue
Type: Single displacement reaction
2AgNO3 + Cu --------> Cu(NO3)2 + 2Ag ↓
Observation: Clear solution of AgNO3 turns Blue
Type: Single displacement reaction
Lemon contains- a)Formic acid
- b)Tartaric acid
- c)Lactic acid
- d)citric acid
Correct answer is option 'D'. Can you explain this answer?
Lemon contains
a)
Formic acid
b)
Tartaric acid
c)
Lactic acid
d)
citric acid

|
Nimansha Singh answered |
D, orange n lemon both contain citric acid
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Periodic Classification of Elements" of Class 10 Science, the questions are available for practice Q. According to IUPAC recommendations, the number of groups in the long form of the periodic table is :-- a)7
- b)8
- c)16
- d)18
Correct answer is option 'D'. Can you explain this answer?
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Periodic Classification of Elements" of Class 10 Science, the questions are available for practice
Q. According to IUPAC recommendations, the number of groups in the long form of the periodic table is :-
a)
7
b)
8
c)
16
d)
18
|
|
Krishna Iyer answered |
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table.
Which non-metal have semi conductor property–- a)Boron
- b)Carbon
- c)Silicon
- d)Magnesium
Correct answer is option 'C'. Can you explain this answer?
Which non-metal have semi conductor property–
a)
Boron
b)
Carbon
c)
Silicon
d)
Magnesium
|
|
Arun Sharma answered |
Silicon is doped with other Microelements to increase its conductivity. It is used in transistors, solar cells, semiconductor devices.
Chapter doubts & questions for Class 10 Chemistry - General Science for Competitive Exams 2025 is part of RRB Group D / RPF Constable exam preparation. The chapters have been prepared according to the RRB Group D / RPF Constable exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for RRB Group D / RPF Constable 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Class 10 Chemistry - General Science for Competitive Exams in English & Hindi are available as part of RRB Group D / RPF Constable exam.
Download more important topics, notes, lectures and mock test series for RRB Group D / RPF Constable Exam by signing up for free.
General Science for Competitive Exams
365 docs|169 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup