All Exams >
Bank Exams >
Crash Course for Banking Exams >
All Questions
All questions of Chemistry for Bank Exams Exam
The ore of Aluminium is - a) Bauxite
- b) Chromium
- c) Mica
- d) Manganese
Correct answer is option 'A'. Can you explain this answer?
The ore of Aluminium is
a)
Bauxite
b)
Chromium
c)
Mica
d)
Manganese
|
|
Amit Kumar answered |
Bauxite, an aluminium ore, is the world’s main source of aluminium. It consists mostly of the minerals gibbsite.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which pair of molecules has the strongest dipole-dipole interactions?- a)NH3 and CH4
- b)CH4 and CH4
- c)CO3 and CO2
- d)NH3 and NH3
Correct answer is 'D'. Can you explain this answer?
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which pair of molecules has the strongest dipole-dipole interactions?
a)
NH3 and CH4
b)
CH4 and CH4
c)
CO3 and CO2
d)
NH3 and NH3
|
|
Naina Bansal answered |
NH3 and NH3. Both polar, asymmetric molecules. CH4 is nonpolar. CO2 is symetrical.
In comparing gases with liquids , gases have ........ compressibility and...........density.- a)greater, smalle
- b)greater, greater
- c)smaller, smaller
- d)smaller, greater
Correct answer is option 'A'. Can you explain this answer?
In comparing gases with liquids , gases have ........ compressibility and...........density.
a)
greater, smalle
b)
greater, greater
c)
smaller, smaller
d)
smaller, greater
|
|
Neha Patel answered |
In a gas, the distance between molecules, whether monatomic or polyatomic, is very large compared with the size of the molecules; thus gases have a low density and are highly compressible.Density: The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases.
Hydrogen bonding reduces the quality of water molecules to
- a)repel
- b)attract
- c)compactly arrange
- d)slide over each other
Correct answer is option 'D'. Can you explain this answer?
Hydrogen bonding reduces the quality of water molecules to
a)
repel
b)
attract
c)
compactly arrange
d)
slide over each other
|
|
Shreya Gupta answered |
Hydrogen bonding is a type of attractive force that occurs between molecules when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In water molecules, hydrogen bonding occurs between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another water molecule. These hydrogen bonds cause the water molecules to attract each other and stick together, which gives water many of its unique properties, such as its high surface tension and its ability to act as a solvent. The hydrogen bonds do not cause the water molecules to repel each other or to compactly arrange, but they do make it more difficult for the molecules to slide over each other, which contributes to the high viscosity of water.
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of  Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is- a)London interaction
- b)dipole-dipole interaction
- c)hydrogen bonding
- d)dipole-induced dipole interaction
Correct answer is option 'A'. Can you explain this answer?
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
a)
London interaction
b)
dipole-dipole interaction
c)
hydrogen bonding
d)
dipole-induced dipole interaction
|
|
Rajeev Saxena answered |
(a) HF - Hydrogen bonding HCl,HBr,HI→ Dipole-dipole interaction, London-dispersion force.
The Defense Research Development Organisation (DRDO) has developed a drug named ‘Lukosin’. It will be used in the treatment of- a)Leukemia
- b)Lucoderma
- c)Lung cancer
- d)Brain tumor
Correct answer is option 'B'. Can you explain this answer?
The Defense Research Development Organisation (DRDO) has developed a drug named ‘Lukosin’. It will be used in the treatment of
a)
Leukemia
b)
Lucoderma
c)
Lung cancer
d)
Brain tumor

|
Rajdeep Roy answered |
Lukosin is a herbal drug developed by Defense Research Development Organisation(DRDO) for treatment of Lucoderma( White patches on skin).
Based on the following statements I and IS, select the correct answer from the codes given.Statement I Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.Statement IIIntermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement il is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Based on the following statements I and IS, select the correct answer from the codes given.
Statement I
Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.
Statement II
Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement il is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Geetika Shah answered |
Thermal energy is the energy of a body arising from motion of its atoms or molecules. It is directly proportional to the temperature of the substance. It is the measure of average kinetic energy of the particles of the matter and is thus responsible for movement of particles. This movement of particles is called thermal motion. We have already learnt that intermolecular forces tend to keep the molecules together but thermal energy of the molecules tends to keep them apart. Three states of matter are the result of balance between intermolecular forces and the thermal energy of the molecules.
Diamond is harder than graphite because of –- a)difference in layers of atoms
- b)tetrahedral structure of diamond
- c)difference of crystalline structures
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Diamond is harder than graphite because of –
a)
difference in layers of atoms
b)
tetrahedral structure of diamond
c)
difference of crystalline structures
d)
None of these
|
|
Eshaan Kapoor answered |
Diamond is harder than graphite because diamond has a more complex structure. Diamond's structure is like many pentagons connected together, each pentagon sharing a side with another pentagon or each pentagon sharing a point with another pentagon. All the points are linked together in some way. Graphite's structure is very loose, with its bonds forming layers.
Atom which must be present in hydrogen bonding is- a)hydrogen
- b)sodium
- c)calcium
- d)sulphur
Correct answer is option 'A'. Can you explain this answer?
Atom which must be present in hydrogen bonding is
a)
hydrogen
b)
sodium
c)
calcium
d)
sulphur
|
|
Nandini Patel answered |
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually the electronegative atom is oxygen, nitrogen, or fluorine, which has a partial negative charge. The hydrogen then has the partial positive charge.
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are- a)

- b)

- c)
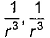
- d)

Correct answer is option 'B'. Can you explain this answer?
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are
a)
b)
c)
d)
|
|
Preeti Iyer answered |
The attractive force decreases with the increase of distance between the dipoles. The interaction energy is inversely proportional to distance between polar molecules. Dipole-dipole interaction energy between stationary polar molecules is proportional to 1/ r^3 and that between rotating polar molecules is proportional to 1/ r^6 where ‘r’ is the distance between polar molecules. Besides dipole - dipole interaction, polar molecules can interact by London forces also Thus cumulative effect is that total intermolecular forces in polar molecules increase.
Which one of the following pairs is not correctly matched?- a)Arjun : Indigenously produced Main Battle Tank (MBT)
- b)Phalcon : Cruise missile supplied by Russia to India.
- c)Saras : Indigenously developed civilian passenger aircraft.
- d)Operation Seabird: New Indian naval base at Karwar.
Correct answer is option 'B'. Can you explain this answer?
Which one of the following pairs is not correctly matched?
a)
Arjun : Indigenously produced Main Battle Tank (MBT)
b)
Phalcon : Cruise missile supplied by Russia to India.
c)
Saras : Indigenously developed civilian passenger aircraft.
d)
Operation Seabird: New Indian naval base at Karwar.

|
Juhi Patel answered |
Phalcon is radar system provided by Israel to India.
Which mineral is mined in Turamdih?- a) Kyanite
- b) Asbestos
- c) Mica
- d) Uranium
Correct answer is option 'D'. Can you explain this answer?
Which mineral is mined in Turamdih?
a)
Kyanite
b)
Asbestos
c)
Mica
d)
Uranium
|
|
Kavita Mehta answered |
- Turamdih Uranium deposit is located about 24 km west of Jaduguda.
Bishrampur is famous for the mining of - a) Copper ore
- b) Iron ore
- c) Coal
- d) Manganese
Correct answer is option 'C'. Can you explain this answer?
Bishrampur is famous for the mining of
a)
Copper ore
b)
Iron ore
c)
Coal
d)
Manganese
|
|
Vijay Kumar answered |
Chhattisgarh is the second most coal-producing state. It has 15% coal quantity of the country while it produces 16% coal in the country. The main coalfield of North Chhattisgarh includes Chirmiri, Kursia Bishrampur, Ghilmili, Sonhat, Lakhanpur Sendouorgarh and Ramkola.
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon- a)charge of interacting particles
- b)mass of interacting particles
- c)strength of permanent dipoles in the particles
- d)polarisability of interacting particles
Correct answer is option 'D'. Can you explain this answer?
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
a)
charge of interacting particles
b)
mass of interacting particles
c)
strength of permanent dipoles in the particles
d)
polarisability of interacting particles
|
|
Rajat Kapoor answered |
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon. (d) strength of permanent dipoles in the particles.
Which of the following elements replaced eka-Aluminium in Mendeleev's Periodic Table?- a)Scandium
- b)Gallium
- c)Titanium
- d)Germanium
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements replaced eka-Aluminium in Mendeleev's Periodic Table?
a)
Scandium
b)
Gallium
c)
Titanium
d)
Germanium
|
|
Sheikha Shah answered |
Answer :
Gallium
Gallium replaced eka-Aluminium in Mendeleev's Periodic Table.
Van der waals forces include the following except
- a)London forces
- b)dipole - dipole forces
- c)dipole- include dipole forces
- d)chemical bonding forces
Correct answer is option 'D'. Can you explain this answer?
Van der waals forces include the following except
a)
London forces
b)
dipole - dipole forces
c)
dipole- include dipole forces
d)
chemical bonding forces
|
|
Om Desai answered |
Chemical bonding forces are not considered to be part of van der Waals forces. Van der Waals forces include London forces, dipole-dipole forces, and dipole-induced dipole forces.
Intermolecular forces can be out of the following.- a)van der Waais' forces
- b)Electrostatic forces existing between two oppositely charged ions
- c)Covalent bond between two like atoms
- d)Gravitational force
Correct answer is option 'A'. Can you explain this answer?
Intermolecular forces can be out of the following.
a)
van der Waais' forces
b)
Electrostatic forces existing between two oppositely charged ions
c)
Covalent bond between two like atoms
d)
Gravitational force
|
|
Shreya Gupta answered |
In molecular physics, the van der Waals forces, named after Dutch scientist Johannes Diderik van der Waals, are distance-dependent interactions between atoms or molecules. Unlike ionic or covalent bonds, these attractions are not a result of any chemical electronic bond, and they are comparatively weak and more susceptible to being perturbed. Van der Waals forces quickly vanish at longer distances between interacting molecules.
Van der Waals forces play a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. Van der Waals forces also define many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media.
Which type of rocks in India produces manganese? - a) Gondwana
- b) Dharwar
- c) Vindhya
- d) Tertiary
Correct answer is option 'B'. Can you explain this answer?
Which type of rocks in India produces manganese?
a)
Gondwana
b)
Dharwar
c)
Vindhya
d)
Tertiary
|
|
Deepa Iyer answered |
The rocks of the Dharwar system are mainly sedimentary in origin and occur in narrow elongated synclines resting on the gneisses found in Bellary district, Mysore and the Aravallis of Rajputana. These rocks are enriched in manganese and iron ore which represents a significant resource of these metals.
Which gas is used to manufacture vanaspati from vegetable oil is -- a)carbon dioxide
- b)nitrogen
- c)oxygen
- d)hydrogen
Correct answer is option 'D'. Can you explain this answer?
Which gas is used to manufacture vanaspati from vegetable oil is -
a)
carbon dioxide
b)
nitrogen
c)
oxygen
d)
hydrogen
|
|
Dia Mehta answered |
Hydrogen is used to manufacture vanaspati from vegetable oil.
Which one amongst the following is not a Green House gas?- a)Nitrogen
- b)Carbon dioxide
- c)Carbon Monoxide
- d)Chloro fluoro carbons
Correct answer is option 'A'. Can you explain this answer?
Which one amongst the following is not a Green House gas?
a)
Nitrogen
b)
Carbon dioxide
c)
Carbon Monoxide
d)
Chloro fluoro carbons
|
|
Dia Mehta answered |
A greenhouse has (sometimes abbreviated GHG) is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. The primary greenhouse gases in the Earth's atmosphere are water vapour, carbon dioxide, methane, nitrous oxide, and ozone.
The element used in the manufacture of safety matches is –- a)Phosphorous
- b)Magnesium
- c)Silicon
- d)Sulphur
Correct answer is option 'A'. Can you explain this answer?
The element used in the manufacture of safety matches is –
a)
Phosphorous
b)
Magnesium
c)
Silicon
d)
Sulphur
|
|
Dia Mehta answered |
One end of a match is coated with a material that can be ignited by frictional heat generated by striking the match against a suitable surface. The coated end of a match, known as the match "head," contains either phosphorus or phosphorus sesquisulfide as the active ingredient and gelatin as a binder.
Urea is a -- a)Sodium fertilizer
- b)Phosphatic fertilizer
- c)Nitrogenous fertilizer
- d)Potassium fertilizer
Correct answer is option 'C'. Can you explain this answer?
Urea is a -
a)
Sodium fertilizer
b)
Phosphatic fertilizer
c)
Nitrogenous fertilizer
d)
Potassium fertilizer
|
|
Faizan Khan answered |
More than 90% of world production of urea is destined for use as a nitrogen-release fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use.
Which one among the following polymers is used for making bullet-proof material?- a)Polyvinyl chloride
- b)Polystyrene
- c)Polyethylene
- d)Polyamide
Correct answer is option 'C'. Can you explain this answer?
Which one among the following polymers is used for making bullet-proof material?
a)
Polyvinyl chloride
b)
Polystyrene
c)
Polyethylene
d)
Polyamide
|
|
Eshaan Kapoor answered |
A bullet-proof material is made of polyethylene. It is a higher grade of the plastic found in Tupperware.
The stages of India’s Nuclear Power Programme differs with respect to:
1. Fuel used
2. Technology
3. Stage of development- a)1 and 2
- b)2 and 3
- c)Only 3
- d)All
Correct answer is option 'D'. Can you explain this answer?
The stages of India’s Nuclear Power Programme differs with respect to:
1. Fuel used
2. Technology
3. Stage of development
1. Fuel used
2. Technology
3. Stage of development
a)
1 and 2
b)
2 and 3
c)
Only 3
d)
All
|
|
Debolina Yadav answered |
's history can be broadly categorized as follows:
1. Indus Valley Civilization (3300 BCE - 1300 BCE): This was one of the earliest civilizations in the world, located in the northwestern region of the Indian subcontinent.
2. Vedic Period (1500 BCE - 500 BCE): This period is characterized by the composition of the Rigveda, the oldest of the four Vedas, and the emergence of Hinduism.
3. Maurya Empire (322 BCE - 185 BCE): This was the first empire to unite most of the Indian subcontinent under one ruler, Emperor Ashoka.
4. Gupta Empire (320 CE - 550 CE): This was a period of great cultural and scientific achievements, known as the "Golden Age" of India.
5. Islamic Sultanates (1206 CE - 1526 CE): This period saw the arrival of Islam in India and the establishment of various Islamic sultanates in different parts of the country.
6. Mughal Empire (1526 CE - 1857 CE): This was a period of great artistic and architectural achievements, but also marked by political instability and conflict with the British.
7. British Raj (1858 CE - 1947 CE): This was a period of direct British rule over India, marked by economic exploitation, social reform movements, and the struggle for independence.
8. Independent India (1947 CE - present): This period marks the establishment of the Republic of India, with a democratic government and a mixed economy. India has continued to make progress in various fields such as science, technology, and social development.
1. Indus Valley Civilization (3300 BCE - 1300 BCE): This was one of the earliest civilizations in the world, located in the northwestern region of the Indian subcontinent.
2. Vedic Period (1500 BCE - 500 BCE): This period is characterized by the composition of the Rigveda, the oldest of the four Vedas, and the emergence of Hinduism.
3. Maurya Empire (322 BCE - 185 BCE): This was the first empire to unite most of the Indian subcontinent under one ruler, Emperor Ashoka.
4. Gupta Empire (320 CE - 550 CE): This was a period of great cultural and scientific achievements, known as the "Golden Age" of India.
5. Islamic Sultanates (1206 CE - 1526 CE): This period saw the arrival of Islam in India and the establishment of various Islamic sultanates in different parts of the country.
6. Mughal Empire (1526 CE - 1857 CE): This was a period of great artistic and architectural achievements, but also marked by political instability and conflict with the British.
7. British Raj (1858 CE - 1947 CE): This was a period of direct British rule over India, marked by economic exploitation, social reform movements, and the struggle for independence.
8. Independent India (1947 CE - present): This period marks the establishment of the Republic of India, with a democratic government and a mixed economy. India has continued to make progress in various fields such as science, technology, and social development.
The name of the new sexually transmitted sexsuperbug, which can be much more deadly than AIDS virus is _____- a)HO41
- b)H41O
- c)H14O
- d)HO14
Correct answer is option 'A'. Can you explain this answer?
The name of the new sexually transmitted sexsuperbug, which can be much more deadly than AIDS virus is _____
a)
HO41
b)
H41O
c)
H14O
d)
HO14

|
Simran Sarkar answered |
One causal virus of gonorrhea has been detected which is more aggressive than HIV in spreading AIDS and is resistance to any antibiotic. Although no infected persons are yet detected with this virus.
What is Nuclear transmutation?- a)Conversion of one chemical element or isotope into another.
- b)Conversion of solid directly into gas
- c)Conversion of gas directly into solid
- d)Conversion of a nucleated human nerve cell into a non-nucleated one
Correct answer is option 'A'. Can you explain this answer?
What is Nuclear transmutation?
a)
Conversion of one chemical element or isotope into another.
b)
Conversion of solid directly into gas
c)
Conversion of gas directly into solid
d)
Conversion of a nucleated human nerve cell into a non-nucleated one

|
Amrutha Kapoor answered |
Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of another element through nuclear reactions or through radioactive decay.
In which one of the following is a higher percentage of carbon found? - a) Lignite coal
- b) Peat coal
- c) Bituminous coal
- d) Anthracite coal
Correct answer is option 'D'. Can you explain this answer?
In which one of the following is a higher percentage of carbon found?
a)
Lignite coal
b)
Peat coal
c)
Bituminous coal
d)
Anthracite coal
|
|
Amit Kumar answered |
Anthracite has a higher percentage of Carbon. It has a carbon content of over 87% on a dry ash-free basis.
Which is the purest form of iron?- a)Steel
- b)Cast iron
- c)Pig iron
- d)Wrought iron
Correct answer is option 'D'. Can you explain this answer?
Which is the purest form of iron?
a)
Steel
b)
Cast iron
c)
Pig iron
d)
Wrought iron
|
|
Aruna singh answered |
Wrought iron is the purest form of iron among the given options.
Explanation:
Wrought iron is a form of iron that is almost entirely free from impurities and contains a very low percentage of carbon (less than 0.1%). It is characterized by its fibrous structure and is known for its high ductility and malleability.
Here are some key points that explain why wrought iron is considered the purest form of iron:
1. Carbon content:
- Wrought iron has the lowest carbon content among the given options. It contains less than 0.1% carbon, making it nearly pure iron.
- In comparison, steel contains a higher carbon content (up to 2%) which gives it greater strength but reduces its purity.
- Cast iron contains a higher carbon content (2-4%) and is known for its brittleness and inability to be forged.
- Pig iron is the crudest form of iron and has a high carbon content (3-4%). It is also brittle and cannot be used directly.
2. Impurities:
- Wrought iron is produced by a refining process known as puddling, which removes impurities such as sulfur and phosphorus.
- This process involves melting pig iron in a reverberatory furnace and stirring it to oxidize the impurities, which are then absorbed by the slag.
- The repeated stirring and oxidation help to purify the iron, resulting in wrought iron with minimal impurities.
3. Physical properties:
- Wrought iron is known for its fibrous structure, which is a result of the slag being worked into the iron during the refining process.
- This fibrous structure gives wrought iron its characteristic grainy appearance and enhances its mechanical properties, such as ductility and malleability.
- It can be easily forged, welded, and shaped into various forms without losing its strength.
In conclusion, wrought iron is considered the purest form of iron due to its low carbon content and the removal of impurities during the refining process. Its high ductility, malleability, and nearly pure iron composition make it suitable for various applications, including decorative ironwork, fencing, and historical artifacts.
Explanation:
Wrought iron is a form of iron that is almost entirely free from impurities and contains a very low percentage of carbon (less than 0.1%). It is characterized by its fibrous structure and is known for its high ductility and malleability.
Here are some key points that explain why wrought iron is considered the purest form of iron:
1. Carbon content:
- Wrought iron has the lowest carbon content among the given options. It contains less than 0.1% carbon, making it nearly pure iron.
- In comparison, steel contains a higher carbon content (up to 2%) which gives it greater strength but reduces its purity.
- Cast iron contains a higher carbon content (2-4%) and is known for its brittleness and inability to be forged.
- Pig iron is the crudest form of iron and has a high carbon content (3-4%). It is also brittle and cannot be used directly.
2. Impurities:
- Wrought iron is produced by a refining process known as puddling, which removes impurities such as sulfur and phosphorus.
- This process involves melting pig iron in a reverberatory furnace and stirring it to oxidize the impurities, which are then absorbed by the slag.
- The repeated stirring and oxidation help to purify the iron, resulting in wrought iron with minimal impurities.
3. Physical properties:
- Wrought iron is known for its fibrous structure, which is a result of the slag being worked into the iron during the refining process.
- This fibrous structure gives wrought iron its characteristic grainy appearance and enhances its mechanical properties, such as ductility and malleability.
- It can be easily forged, welded, and shaped into various forms without losing its strength.
In conclusion, wrought iron is considered the purest form of iron due to its low carbon content and the removal of impurities during the refining process. Its high ductility, malleability, and nearly pure iron composition make it suitable for various applications, including decorative ironwork, fencing, and historical artifacts.
The fuel that is used in modern submarines is –- a)Nuclear fuel
- b)Petrol
- c)Coal
- d)Diesel
Correct answer is option 'A'. Can you explain this answer?
The fuel that is used in modern submarines is –
a)
Nuclear fuel
b)
Petrol
c)
Coal
d)
Diesel
|
|
Dia Mehta answered |
Nuclear power is now used in all large submarines, but due to the high cost and large size of nuclear reactors, smaller submarines still use diesel-electric propulsion. The ratio of larger to smaller submarines depends on strategic needs. The US Navy, drench Navy, and the British Royal Navy operate only nuclear submarines.
Manganite is an ore/mineral of ______.- a)Beryllium
- b)Chromium
- c)Manganese
- d)Copper
Correct answer is option 'C'. Can you explain this answer?
Manganite is an ore/mineral of ______.
a)
Beryllium
b)
Chromium
c)
Manganese
d)
Copper
|
|
Aryan Khanna answered |
Manganite is an ore mineral of manganese. As a manganese ore, it ranks after pyrolusite and romanechite.
In vulcanisation process, rubber can be hardened by adding -- a)Nitrogen
- b)Silicon
- c)Sulphur
- d)Alcohol
Correct answer is option 'C'. Can you explain this answer?
In vulcanisation process, rubber can be hardened by adding -
a)
Nitrogen
b)
Silicon
c)
Sulphur
d)
Alcohol
|
|
Eshaan Kapoor answered |
In order to give more strength and more elasticity, natural rubber is heated with sulphur or sulphur compounds at 150°C temperature. Vulcanized rubber has good tensile strength.
Quartz is a crystalline form of -- a)Alumina
- b)Glass
- c)Silica
- d)Limestone
Correct answer is option 'C'. Can you explain this answer?
Quartz is a crystalline form of -
a)
Alumina
b)
Glass
c)
Silica
d)
Limestone
|
|
Dia Mehta answered |
Quartz is made up of a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. Tridymite and cristobalite are hightemperature polymorphs of SiO2 that occur in high-silica volcanic rocks. Coesite is a denser polymorph of quartz found in some meteorite impact sites and in metamorphic rocks.
A body moving in a circular path with a constant speed has a -- a)constant velocity
- b)constant acceleration
- c)constant kinetic energy
- d)constant displacement
Correct answer is option 'C'. Can you explain this answer?
A body moving in a circular path with a constant speed has a -
a)
constant velocity
b)
constant acceleration
c)
constant kinetic energy
d)
constant displacement
|
|
Shradha nayar answered |
Understanding Circular Motion
When a body moves in a circular path at a constant speed, it exhibits unique characteristics due to its continuous change in direction. Let's explore why the correct answer is constant kinetic energy.
Constant Speed vs. Velocity
- Speed vs. Velocity: Speed is a scalar quantity, meaning it only has magnitude. In contrast, velocity is a vector quantity, which has both magnitude and direction.
- Changing Direction: Although the speed is constant, the direction of the velocity vector changes continuously, making the velocity itself non-constant.
Acceleration in Circular Motion
- Centripetal Acceleration: A body moving in a circular path experiences centripetal acceleration directed towards the center of the circle.
- Constant Acceleration: While this acceleration is constant in magnitude, its direction changes, indicating that it is not constant in the conventional sense.
Constant Kinetic Energy
- Kinetic Energy Formula: Kinetic energy (KE) is given by the formula KE = 1/2 mv², where m is mass and v is speed.
- Impact of Constant Speed: Since the speed remains unchanged during circular motion, the kinetic energy of the body also remains constant.
- Independence from Direction: Kinetic energy does not depend on the direction of motion, only on the speed.
Displacement in Circular Motion
- Variable Displacement: While moving in a circular path, the displacement (the shortest distance between the initial and final points) changes continuously.
- Cyclic Nature: After one complete revolution, the displacement is zero, further indicating that it is not constant.
In summary, while the body moving in a circular path has constant speed and kinetic energy, its velocity, acceleration, and displacement vary. Thus, the correct answer is option 'C' - constant kinetic energy.
When a body moves in a circular path at a constant speed, it exhibits unique characteristics due to its continuous change in direction. Let's explore why the correct answer is constant kinetic energy.
Constant Speed vs. Velocity
- Speed vs. Velocity: Speed is a scalar quantity, meaning it only has magnitude. In contrast, velocity is a vector quantity, which has both magnitude and direction.
- Changing Direction: Although the speed is constant, the direction of the velocity vector changes continuously, making the velocity itself non-constant.
Acceleration in Circular Motion
- Centripetal Acceleration: A body moving in a circular path experiences centripetal acceleration directed towards the center of the circle.
- Constant Acceleration: While this acceleration is constant in magnitude, its direction changes, indicating that it is not constant in the conventional sense.
Constant Kinetic Energy
- Kinetic Energy Formula: Kinetic energy (KE) is given by the formula KE = 1/2 mv², where m is mass and v is speed.
- Impact of Constant Speed: Since the speed remains unchanged during circular motion, the kinetic energy of the body also remains constant.
- Independence from Direction: Kinetic energy does not depend on the direction of motion, only on the speed.
Displacement in Circular Motion
- Variable Displacement: While moving in a circular path, the displacement (the shortest distance between the initial and final points) changes continuously.
- Cyclic Nature: After one complete revolution, the displacement is zero, further indicating that it is not constant.
In summary, while the body moving in a circular path has constant speed and kinetic energy, its velocity, acceleration, and displacement vary. Thus, the correct answer is option 'C' - constant kinetic energy.
What is "milk of magnesia" chemically?- a)Magnesium carbonate
- b)Sodium bicarbonate
- c)Calcium hydroxide
- d)Magnesium hydroxide
Correct answer is option 'D'. Can you explain this answer?
What is "milk of magnesia" chemically?
a)
Magnesium carbonate
b)
Sodium bicarbonate
c)
Calcium hydroxide
d)
Magnesium hydroxide
|
|
Anaya Patel answered |
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. As a suspension in water, it is often called milk of magnesia because of its milk-like appearance. The solid mineral form of magnesium hydroxide is known as brucite.
The largest reserve of crude oil in India is found in - a) Assam
- b) Gujarat
- c) Eastern offshore
- d) Western offshore
Correct answer is option 'D'. Can you explain this answer?
The largest reserve of crude oil in India is found in
a)
Assam
b)
Gujarat
c)
Eastern offshore
d)
Western offshore
|
|
Anshu Khanna answered |
As of 31 March 2019, India had estimated crude oil reserves of 618.95 million tonnes (MT), increasing by 4.1% from the previous year. The largest reserves are found in the Western Offshore (Mumbai High, Krishna-Godavari Basin) (40%), and Assam (27%).
The pH of a neutral solution is -- a)0-7
- b)7
- c)7-14
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The pH of a neutral solution is -
a)
0-7
b)
7
c)
7-14
d)
None of the above
|
|
Dia Mehta answered |
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, the solution turns more basic.
Direction (Q. Nos. 7-10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct alternate(s).
- a)Gases can be compressed easily
- b)Lack of compressibility is a characteristic property of liquids and solids
- c)Oxygen can dissolve In water because a force of attraction exists between water's perm anent dipoie moment and the induced dipole in O2
- d)London dispersion forces are the only intermolecular forces that allow non-poiar molecules to interact
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Direction (Q. Nos. 7-10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct alternate(s).
a)
Gases can be compressed easily
b)
Lack of compressibility is a characteristic property of liquids and solids
c)
Oxygen can dissolve In water because a force of attraction exists between water's perm anent dipoie moment and the induced dipole in O2
d)
London dispersion forces are the only intermolecular forces that allow non-poiar molecules to interact
|
|
Jithin Saini answered |
All of the above are the properties of gases.
The gas usually causing explosions in coal mines is –- a)Hydrogen
- b)Carbon monoxide
- c)Air
- d)Methane
Correct answer is option 'D'. Can you explain this answer?
The gas usually causing explosions in coal mines is –
a)
Hydrogen
b)
Carbon monoxide
c)
Air
d)
Methane
|
|
Dia Mehta answered |
Firedamp is flammable gas found in coal mines. It is the name given to a number of flammable gases, especially methane.
Who discovered the atom bomb?- a)Madam Curie
- b)Pierre Curie
- c)Otto Hahn
- d)Albert Einstein
Correct answer is option 'C'. Can you explain this answer?
Who discovered the atom bomb?
a)
Madam Curie
b)
Pierre Curie
c)
Otto Hahn
d)
Albert Einstein
|
|
Aryan Khanna answered |
Otto Hahn, (8 March, 1879 - 28 July, 1968) was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". On 15 November 1945 the Royal Swedish Academy of Sciences announced that Hahn had been awarded the 1944 Nobel Prize in Chemistry "for his discovery of the fission of heavy atomic nuclei." Otto Hahn received many governmental honours and academic awards from all over the world for his scientific work.
Which of the following is a physical change?- a)oxidation
- b)reduction
- c)sublimation
- d)decomposition
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a physical change?
a)
oxidation
b)
reduction
c)
sublimation
d)
decomposition
|
|
Faizan Khan answered |
Sublimation is a type of phase transition, or a change in a state of matter, just like melting, freezing, and evaporation.
Commercial Vaseline is derived from -- a)plant gums
- b)coal tar
- c)wool wax
- d)petroleum
Correct answer is option 'D'. Can you explain this answer?
Commercial Vaseline is derived from -
a)
plant gums
b)
coal tar
c)
wool wax
d)
petroleum
|
|
Faizan Khan answered |
Vaseline is a brand of petroleum jelly based products owned by Anglo-Dutch company Unilever. While Vaseline can be used as a lubricant, it is also a useful moisture insulator for local skin conditions characterized by tissue dehydration. Vaseline helps protect minor cuts and burns.
Where do most of the known asteroids orbit the Sun?- a)Between the orbits of Mars and Jupiter
- b)Between the orbits of Venus and Earth
- c)Between the orbits of Earth and Mars
- d)Between the orbits of Pluto and Saturn
Correct answer is option 'A'. Can you explain this answer?
Where do most of the known asteroids orbit the Sun?
a)
Between the orbits of Mars and Jupiter
b)
Between the orbits of Venus and Earth
c)
Between the orbits of Earth and Mars
d)
Between the orbits of Pluto and Saturn

|
Deepika Gupta answered |
The asteroid belt is a doughnut-shaped concentration of many different sized asteroids between the orbits of Mars and Jupiter, closer to the orbit of Mars. The asteroids orbit the Sun. The asteroid belt is not smooth but there are concentric gaps in it known as Kirkwood gaps.
Enriched uranium is one in which?- a)Percentage of 235U has been artificially increased
- b)Percentage of U has been artificially increased
- c)Percentage of 234U has been artificially increased
- d)Extra energy is pumped from outside
Correct answer is option 'A'. Can you explain this answer?
Enriched uranium is one in which?
a)
Percentage of 235U has been artificially increased
b)
Percentage of U has been artificially increased
c)
Percentage of 234U has been artificially increased
d)
Extra energy is pumped from outside
|
|
Madhurima Tiwari answered |
Enriched uranium is one in which the percentage of 235U has been artificially increased.
Explanation:
Enriched uranium refers to a type of uranium in which the percentage of the isotope uranium-235 (235U) has been artificially increased. Uranium occurs naturally in the Earth's crust and is composed of three isotopes: uranium-238 (238U), uranium-235 (235U), and uranium-234 (234U). These isotopes have slightly different atomic masses due to variations in the number of neutrons in their nuclei.
Why is uranium enrichment necessary?
Uranium enrichment is necessary for several applications, including nuclear power generation and the production of nuclear weapons. Natural uranium as found in nature typically contains only about 0.7% uranium-235, while the majority is uranium-238. In order to use uranium as fuel in nuclear reactors or to create weapons-grade material, it is necessary to increase the concentration of uranium-235 through a process called enrichment.
The Uranium Enrichment Process:
The process of uranium enrichment involves increasing the concentration of uranium-235 by separating it from uranium-238. There are various methods of enrichment, but the most common one is centrifuge enrichment.
Centrifuge Enrichment:
In centrifuge enrichment, uranium hexafluoride (UF6) gas is fed into a series of high-speed centrifuges. These centrifuges spin at extremely high speeds, causing the heavier uranium-238 to migrate towards the outer edge while the lighter uranium-235 collects near the center. This separation is based on the slight difference in mass between the two isotopes.
Gradual Increase in Uranium-235 Concentration:
Through multiple stages of centrifuge enrichment, the concentration of uranium-235 can be gradually increased. The enriched uranium produced through this process is then used as fuel in nuclear power plants or can be further processed for military purposes.
Conclusion:
Enriched uranium is a type of uranium in which the percentage of uranium-235 has been artificially increased. This is achieved through a process called uranium enrichment, where the heavier uranium-238 is separated from the lighter uranium-235. Enriched uranium is used in various applications, including nuclear power generation and the production of nuclear weapons.
Explanation:
Enriched uranium refers to a type of uranium in which the percentage of the isotope uranium-235 (235U) has been artificially increased. Uranium occurs naturally in the Earth's crust and is composed of three isotopes: uranium-238 (238U), uranium-235 (235U), and uranium-234 (234U). These isotopes have slightly different atomic masses due to variations in the number of neutrons in their nuclei.
Why is uranium enrichment necessary?
Uranium enrichment is necessary for several applications, including nuclear power generation and the production of nuclear weapons. Natural uranium as found in nature typically contains only about 0.7% uranium-235, while the majority is uranium-238. In order to use uranium as fuel in nuclear reactors or to create weapons-grade material, it is necessary to increase the concentration of uranium-235 through a process called enrichment.
The Uranium Enrichment Process:
The process of uranium enrichment involves increasing the concentration of uranium-235 by separating it from uranium-238. There are various methods of enrichment, but the most common one is centrifuge enrichment.
Centrifuge Enrichment:
In centrifuge enrichment, uranium hexafluoride (UF6) gas is fed into a series of high-speed centrifuges. These centrifuges spin at extremely high speeds, causing the heavier uranium-238 to migrate towards the outer edge while the lighter uranium-235 collects near the center. This separation is based on the slight difference in mass between the two isotopes.
Gradual Increase in Uranium-235 Concentration:
Through multiple stages of centrifuge enrichment, the concentration of uranium-235 can be gradually increased. The enriched uranium produced through this process is then used as fuel in nuclear power plants or can be further processed for military purposes.
Conclusion:
Enriched uranium is a type of uranium in which the percentage of uranium-235 has been artificially increased. This is achieved through a process called uranium enrichment, where the heavier uranium-238 is separated from the lighter uranium-235. Enriched uranium is used in various applications, including nuclear power generation and the production of nuclear weapons.
Consider the following statements about the miningindustry of India1. The spatial distribution of minerals is uneven.2. The mining industry since colonial days has been export-oriented.Which of the statements given above is/are correct?- a) Only 1
- b) Only 2
- c) Both 1 and 2
- d) Neither 1 nor 2
Correct answer is option 'C'. Can you explain this answer?
Consider the following statements about the mining
industry of India
1. The spatial distribution of minerals is uneven.
2. The mining industry since colonial days has been export-oriented.
Which of the statements given above is/are correct?
a)
Only 1
b)
Only 2
c)
Both 1 and 2
d)
Neither 1 nor 2
|
|
Sneha Shah answered |
Uneven Spatial Distribution of Minerals:
- India has a rich mineral resource base with vast deposits of coal, iron ore, bauxite, manganese, and other minerals.
- However, the distribution of these minerals across the country is uneven, with certain states like Jharkhand, Odisha, Chhattisgarh, and Karnataka having significant mineral reserves, while others may have limited resources.
- This non-uniform distribution of minerals has led to disparities in economic development and industrial growth across different regions of the country.
Export-Oriented Mining Industry:
- The mining industry in India has historically been export-oriented, with a focus on extracting and exporting raw minerals to other countries.
- During the colonial era, the British exploited India's mineral resources primarily for export to meet the demands of their industries back home.
- Even after independence, India continued to export minerals like iron ore, bauxite, and coal to countries like China, Japan, and South Korea to generate foreign exchange earnings.
Conclusion:
- Both statements are correct as the uneven spatial distribution of minerals in India has influenced the development of the mining industry, which has traditionally been export-oriented.
- It is essential for India to focus on sustainable and inclusive development of its mining sector, ensuring equitable distribution of benefits and resources across different regions of the country.
- India has a rich mineral resource base with vast deposits of coal, iron ore, bauxite, manganese, and other minerals.
- However, the distribution of these minerals across the country is uneven, with certain states like Jharkhand, Odisha, Chhattisgarh, and Karnataka having significant mineral reserves, while others may have limited resources.
- This non-uniform distribution of minerals has led to disparities in economic development and industrial growth across different regions of the country.
Export-Oriented Mining Industry:
- The mining industry in India has historically been export-oriented, with a focus on extracting and exporting raw minerals to other countries.
- During the colonial era, the British exploited India's mineral resources primarily for export to meet the demands of their industries back home.
- Even after independence, India continued to export minerals like iron ore, bauxite, and coal to countries like China, Japan, and South Korea to generate foreign exchange earnings.
Conclusion:
- Both statements are correct as the uneven spatial distribution of minerals in India has influenced the development of the mining industry, which has traditionally been export-oriented.
- It is essential for India to focus on sustainable and inclusive development of its mining sector, ensuring equitable distribution of benefits and resources across different regions of the country.
The Refrigerant 'FREON' is –- a)Calcium Tetra Fluoride
- b)Difluoro Dichloro Methane
- c)Fluorspar and Felspar
- d)Hydrofluosilicic Acid
Correct answer is option 'B'. Can you explain this answer?
The Refrigerant 'FREON' is –
a)
Calcium Tetra Fluoride
b)
Difluoro Dichloro Methane
c)
Fluorspar and Felspar
d)
Hydrofluosilicic Acid
|
|
Eshaan Kapoor answered |
Dichlorodifluorornethane (R-12), is a colourless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halornethane (CFC), used as a refrigrant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other countries in 1994 clue to concerns about damage to the ozone layer. It is soluble in many organic solvents.
The major harmful gas emitted by automobile vehicles which causes air pollution is –- a)Carbon Monoxide
- b)Methane
- c)Carbon dioxide
- d)Ozone gas
Correct answer is option 'A'. Can you explain this answer?
The major harmful gas emitted by automobile vehicles which causes air pollution is –
a)
Carbon Monoxide
b)
Methane
c)
Carbon dioxide
d)
Ozone gas
|
|
Eshaan Kapoor answered |
Carbon monoxide is the major harmful gas emitted by the automobile vehicles which causes air pollution. Carbon monoxide (CO) - A product of incomplete combustion, carbon monoxide reduces the blood's ability to carry oxygen: overexposure (carbon monoxide poisoning) may be fatal. Carbon Monoxide poisoning is a major killer.
Which one of the following is a major green gas?- a)Carbon dioxide
- b)Chloro fluorocarbon
- c)Carbon monoxide
- d)Freon
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is a major green gas?
a)
Carbon dioxide
b)
Chloro fluorocarbon
c)
Carbon monoxide
d)
Freon
|
|
Ramya jain answered |
Carbon Dioxide as a Major Green Gas
Carbon dioxide (CO2) is a major greenhouse gas that contributes significantly to global warming and climate change. Here's why it is considered a major green gas:
- Abundance: Carbon dioxide is one of the most abundant greenhouse gases in the Earth's atmosphere, primarily due to human activities such as burning fossil fuels, deforestation, and industrial processes.
- Global Warming Potential: CO2 has a high global warming potential, meaning it has a strong ability to trap heat in the atmosphere and contribute to the greenhouse effect.
- Longevity: Carbon dioxide remains in the atmosphere for a long time, with some of it staying for thousands of years. This long-lasting nature makes it a persistent greenhouse gas.
- Impact on Climate: The excessive accumulation of carbon dioxide in the atmosphere is causing temperatures to rise, ice caps to melt, sea levels to rise, and extreme weather events to become more frequent and severe.
- Regulation: Due to its significant role in climate change, efforts are being made globally to reduce CO2 emissions through policies, agreements, and initiatives aimed at mitigating its impact on the environment.
In conclusion, carbon dioxide is a major greenhouse gas with far-reaching implications for the planet's climate and ecosystems. Addressing the issue of CO2 emissions is crucial in combating global warming and its associated consequences.
Carbon dioxide (CO2) is a major greenhouse gas that contributes significantly to global warming and climate change. Here's why it is considered a major green gas:
- Abundance: Carbon dioxide is one of the most abundant greenhouse gases in the Earth's atmosphere, primarily due to human activities such as burning fossil fuels, deforestation, and industrial processes.
- Global Warming Potential: CO2 has a high global warming potential, meaning it has a strong ability to trap heat in the atmosphere and contribute to the greenhouse effect.
- Longevity: Carbon dioxide remains in the atmosphere for a long time, with some of it staying for thousands of years. This long-lasting nature makes it a persistent greenhouse gas.
- Impact on Climate: The excessive accumulation of carbon dioxide in the atmosphere is causing temperatures to rise, ice caps to melt, sea levels to rise, and extreme weather events to become more frequent and severe.
- Regulation: Due to its significant role in climate change, efforts are being made globally to reduce CO2 emissions through policies, agreements, and initiatives aimed at mitigating its impact on the environment.
In conclusion, carbon dioxide is a major greenhouse gas with far-reaching implications for the planet's climate and ecosystems. Addressing the issue of CO2 emissions is crucial in combating global warming and its associated consequences.
What is the most commonly used substance in fluorescent tubes?- a)Sodium oxide and argon
- b)Sodium vapour and neon
- c)Mercury vapour and argon
- d)Mercury oxide and neon
Correct answer is option 'C'. Can you explain this answer?
What is the most commonly used substance in fluorescent tubes?
a)
Sodium oxide and argon
b)
Sodium vapour and neon
c)
Mercury vapour and argon
d)
Mercury oxide and neon
|
|
Eshaan Kapoor answered |
A fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapour. it contains mercury vapour and argon. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful light more efficiently than an incandescent lamp.
Soap is prepared by boiling caustic soda with –- a)Alcohol
- b)Kerosene oil
- c)Glycerine
- d)Fats
Correct answer is option 'D'. Can you explain this answer?
Soap is prepared by boiling caustic soda with –
a)
Alcohol
b)
Kerosene oil
c)
Glycerine
d)
Fats
|
|
Eshaan Kapoor answered |
The most basic kind of soap is made from cuastic soda and animal fat. The two are heated together, and then cooled. The process is called "saponification". In technical terms, saponification involves base (usually caustic soda NaOH) hydrolysis of triglyeerides which are esters of fatty acids, to form the sodium salt, of a carboxylate.
Chapter doubts & questions for Chemistry - Crash Course for Banking Exams 2024 is part of Bank Exams exam preparation. The chapters have been prepared according to the Bank Exams exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Bank Exams 2024 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry - Crash Course for Banking Exams in English & Hindi are available as part of Bank Exams exam.
Download more important topics, notes, lectures and mock test series for Bank Exams Exam by signing up for free.
Crash Course for Banking Exams
521 videos|355 docs|26 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup