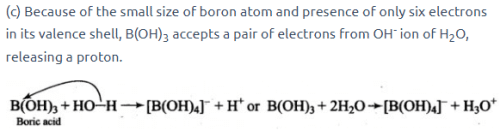All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Classification of Elements and Periodicity in Properties for JAMB Exam
Lothar Meyer proposed that on arranging the elements in order of increasing atomic weights; similarities appear in which type of properties?- a)Only physical properties
- b)Only chemical properties
- c)In both physical and chemical properties
- d)thermodynamic properties
Correct answer is option 'C'. Can you explain this answer?
Lothar Meyer proposed that on arranging the elements in order of increasing atomic weights; similarities appear in which type of properties?
a)
Only physical properties
b)
Only chemical properties
c)
In both physical and chemical properties
d)
thermodynamic properties
|
|
Anoushka Yadav answered |
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. The correct answer is option 'C' which means similarities appear in both physical and chemical properties.
Explanation:
The periodic table arranges the elements in a way that helps in understanding their properties. The modern periodic table is based on the electronic configuration of the elements. But the original periodic table was based on the atomic weight of the elements. Lothar Meyer was the first to realize that there is a periodicity in the properties of the elements when they are arranged in order of increasing atomic weights.
Physical properties are those that can be observed or measured without changing the chemical composition of the substance. Some examples of physical properties are:
- Melting point
- Boiling point
- Density
- Electrical conductivity
- Thermal conductivity
- Atomic radius
- Ionic radius
- Electronegativity
Chemical properties are those that can be observed during a chemical reaction. Some examples of chemical properties are:
- Reactivity with acids
- Reactivity with oxygen
- Reactivity with water
- Reduction potential
- Oxidation potential
Meyer observed that when the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties. This means that elements with similar atomic weights have similar physical and chemical properties. For example, lithium, sodium, and potassium have similar physical and chemical properties because they all belong to the same group and have similar atomic weights.
Conclusion:
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. When the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties.
Explanation:
The periodic table arranges the elements in a way that helps in understanding their properties. The modern periodic table is based on the electronic configuration of the elements. But the original periodic table was based on the atomic weight of the elements. Lothar Meyer was the first to realize that there is a periodicity in the properties of the elements when they are arranged in order of increasing atomic weights.
Physical properties are those that can be observed or measured without changing the chemical composition of the substance. Some examples of physical properties are:
- Melting point
- Boiling point
- Density
- Electrical conductivity
- Thermal conductivity
- Atomic radius
- Ionic radius
- Electronegativity
Chemical properties are those that can be observed during a chemical reaction. Some examples of chemical properties are:
- Reactivity with acids
- Reactivity with oxygen
- Reactivity with water
- Reduction potential
- Oxidation potential
Meyer observed that when the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties. This means that elements with similar atomic weights have similar physical and chemical properties. For example, lithium, sodium, and potassium have similar physical and chemical properties because they all belong to the same group and have similar atomic weights.
Conclusion:
Lothar Meyer proposed the periodic table in 1864. He arranged the elements in order of increasing atomic weights. He noticed that similarities appeared in both physical and chemical properties of the elements. When the elements are arranged in order of increasing atomic weights, there is a periodicity in their physical and chemical properties.
Horizontal rows in the periodic table are called:- a)Cell
- b)Table
- c)Groups
- d)Periods
Correct answer is option 'D'. Can you explain this answer?
Horizontal rows in the periodic table are called:
a)
Cell
b)
Table
c)
Groups
d)
Periods
|
|
Rohan Singh answered |
The Periodic Table: Families and Periods. In the periodic table of elements, there are seven horizontal rows of elements called periods. The vertical columns of elements are called groups, or families.
Can you explain the answer of this question below:In the modern periodic table, which period contains 32 elements?
- A:
Sixth
- B:
First
- C:
Seventh
- D:
Second
The answer is a.
In the modern periodic table, which period contains 32 elements?
Sixth
First
Seventh
Second
|
|
Preeti Iyer answered |
The answer is c.
The total number of electrons that can be accommodated in seventh period are 2 ( in 7s) + 14(in 5f) + 10(in 6d )+ 6(in 7p) = 32. The maximum number of elements present in it is 32.
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:- a)Elements of group 7 and 8 were arranged on the basis of equivalent weights
- b)Some elements in the same group differ in their properties
- c)Elements were arranged on the basis of atomic weights
- d)The position of hydrogen was not justified
Correct answer is option 'A'. Can you explain this answer?
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
a)
Elements of group 7 and 8 were arranged on the basis of equivalent weights
b)
Some elements in the same group differ in their properties
c)
Elements were arranged on the basis of atomic weights
d)
The position of hydrogen was not justified
|
|
Om Desai answered |
In Mendeleev’s periodic table, elements were arranged on the basis of atomic weights.
Which block of the periodic table contains the man made elements?- a)p block
- b)s block
- c)f block
- d)d block
Correct answer is option 'C'. Can you explain this answer?
Which block of the periodic table contains the man made elements?
a)
p block
b)
s block
c)
f block
d)
d block
|
|
Raghav Bansal answered |
Most of the f block elements, lanthanoids and actinoids are man made/synthetic/prepared in the lab. Especially actinoids.
Eka silicon predicted by Mendeleev is which element:- a)Germanium
- b)Aluminium
- c)Gallium
- d)Sodium
Correct answer is option 'A'. Can you explain this answer?
Eka silicon predicted by Mendeleev is which element:
a)
Germanium
b)
Aluminium
c)
Gallium
d)
Sodium
|
|
Suresh Iyer answered |
Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap next to silicon. The element germanium was discovered later. Its properties were found to be similar to the predicted ones and confirmed Mendeleev's periodic table.
Which of the following pairs have both the members from the same group of periodic table?- a)Mg, Cl
- b)Mg, Cu
- c)Mg, Be
- d)Mg, Na
Correct answer is option 'C'. Can you explain this answer?
Which of the following pairs have both the members from the same group of periodic table?
a)
Mg, Cl
b)
Mg, Cu
c)
Mg, Be
d)
Mg, Na
|
|
Neha Joshi answered |
The elements in the group include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?- a)Newlands
- b)Lothar Meyer
- c)Henry Moseley
- d)Meyer
Correct answer is option 'C'. Can you explain this answer?
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?
a)
Newlands
b)
Lothar Meyer
c)
Henry Moseley
d)
Meyer
|
|
Pooja Shah answered |
The scientist who first of all showed that the atomic number is more fundamental property of an element than its atomic mass was Henry Moseley.
Which of the following is the most non-metallic element?- a)F
- b)Si
- c)N
- d)B
Correct answer is option 'A'. Can you explain this answer?
Which of the following is the most non-metallic element?
a)
F
b)
Si
c)
N
d)
B
|
|
Nandini Patel answered |
The non-metallic character increases as we go from left to right across the periodic table. Hence, fluorine (F) is most non-metallic. Further F has maximum tendency to accept an electron characteristic of non-metal.
Which of the following is an ionic hydride?- a)PH3
- b)H2S
- c)HI
- d)KH
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an ionic hydride?
a)
PH3
b)
H2S
c)
HI
d)
KH

|
Mohit Rajpoot answered |
Ionic hydrides are commonly known as saline hydrides or pseudohalides. These compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements.
In this group, the hydrogen acts as the hydride ion (H−). They bond with more electropositive metal atoms. Ionic hydrides are usually binary compounds (i.e., only two elements in the compound) and are also insoluble in solutions. Now I hope you can form your own examples, but common examples are Sodium Hydride (NaH), Lithium hydride (LiH), Potassium hydride (KH) etc.
Eka aluminium predicted by Mendeleev is which element?- a)Germanium
- b)Magnesium
- c)Gallium
- d)Sodium
Correct answer is option 'C'. Can you explain this answer?
Eka aluminium predicted by Mendeleev is which element?
a)
Germanium
b)
Magnesium
c)
Gallium
d)
Sodium
|
|
Gaurav Kumar answered |
Eka aluminium predicted by Mendeleev is Gallium. Eka-aluminium and gallium are the two names of the same element as Eka -Aluminum has almost exactly the same properties as the actual properties of the gallium element.
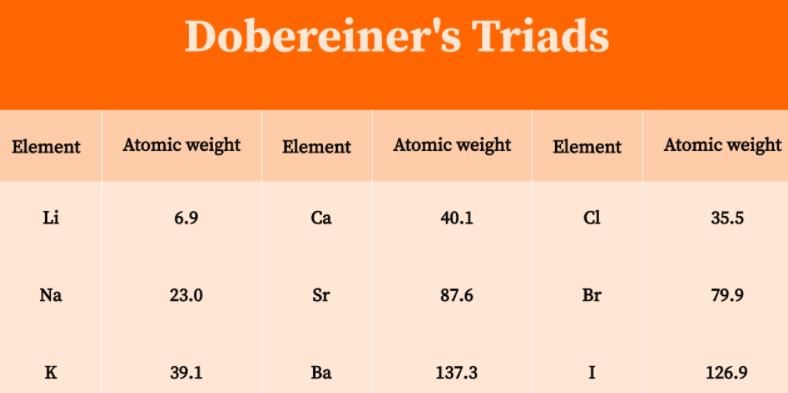
According to Dobereiner’s law of triads the number of elements present in each group is:
- a)2
- b)4
- c)5
- d)3
Correct answer is option 'D'. Can you explain this answer?
According to Dobereiner’s law of triads the number of elements present in each group is:
a)
2
b)
4
c)
5
d)
3
|
|
Geetika Shah answered |
- According to Dobereiner's law of triads each triad contains three elements.
- He also noticed that the middle element of each of the triads had an atomic weight about halfway between the atomic weights of the other two.
The oxygen family is also known as:- a)halogen family
- b)oxo family
- c)chalcogen family
- d)peroxo family
Correct answer is option 'C'. Can you explain this answer?
The oxygen family is also known as:
a)
halogen family
b)
oxo family
c)
chalcogen family
d)
peroxo family
|
|
Rajat Kapoor answered |
The Oxygen family, sometimes also known as chalcogens, is group 16 on the periodic table and consisted of oxygen, sulfur, selenium, tellurium, polonium and ununhexium.
Moseley performed experiments and studied the frequencies of which radiations emitted from the elements?- a)Gamma rays
- b)X-rays
- c)UV rays
- d)Infra red rays
Correct answer is option 'B'. Can you explain this answer?
Moseley performed experiments and studied the frequencies of which radiations emitted from the elements?
a)
Gamma rays
b)
X-rays
c)
UV rays
d)
Infra red rays
|
|
Naina Bansal answered |
Moseley performed experiments and studied the frequencies of the X-rays emitted from the elements.
Johann Dobereiner classified elements in group of three elements called as- a)Trinity
- b)Trials
- c)Triads
- d)Diads
Correct answer is option 'C'. Can you explain this answer?
Johann Dobereiner classified elements in group of three elements called as
a)
Trinity
b)
Trials
c)
Triads
d)
Diads
|
|
Om Desai answered |
In 1829, Johann Dobereiner, a German scientist made some groups of three elements each and called them triads.
He observed that the atomic mass of the middle element of a triad was nearly equal to the arithmetic mean of the atomic masses of the other two elements. All three elements of a triad were similar in their properties.
The transition elements have characteristic electronic configuration which can be represented as:- a)(n-1) d1-10 ns0-2
- b)(n-2) d1-10 (n-1) p6 ns2
- c)(n-1)s2 p6 d 10 ns2 np6 nd1-10
- d)(n-2) d1-10 (n-1) s2 p6 d1 or 2 ns1
Correct answer is option 'A'. Can you explain this answer?
The transition elements have characteristic electronic configuration which can be represented as:
a)
(n-1) d1-10 ns0-2
b)
(n-2) d1-10 (n-1) p6 ns2
c)
(n-1)s2 p6 d 10 ns2 np6 nd1-10
d)
(n-2) d1-10 (n-1) s2 p6 d1 or 2 ns1
|
|
Om Desai answered |
Transition elements are d-block elements and the characteristic electronic configuration of d block element is in option A
Mendeleev predicted the existence of which element/elements in the periodic table?- a)Gallium
- b)Sodium and germanium
- c)Gallium and germanium
- d)Germanium and Gold
Correct answer is option 'C'. Can you explain this answer?
Mendeleev predicted the existence of which element/elements in the periodic table?
a)
Gallium
b)
Sodium and germanium
c)
Gallium and germanium
d)
Germanium and Gold
|
|
Neha Joshi answered |
Gallium and Germanium were the elements not discovered at that time and Mendeleev put gaps in the periodic table.
Gallium was called as Eka aluminium
Germanium was called as Eka silicon
Gallium was called as Eka aluminium
Germanium was called as Eka silicon
For alkali metals, which one of the following trends is INCORRECT?
- a)Hydration energy: Li > Na > K > Rb
- b)Ionization energy: Li > Na > K > Rb
- c)Atomic size: Li < Na < K < Rb
- d)Density: Li < Na < K < Rb
Correct answer is option 'D'. Can you explain this answer?
For alkali metals, which one of the following trends is INCORRECT?
a)
Hydration energy: Li > Na > K > Rb
b)
Ionization energy: Li > Na > K > Rb
c)
Atomic size: Li < Na < K < Rb
d)
Density: Li < Na < K < Rb
|
|
Om Desai answered |
Density: Li < Na < K < Rb.
The density of K is lower than that of Na. Thus, option D is incorrect. The correct trend is
Li < K < Na < Rb
The density of K is lower than that of Na. Thus, option D is incorrect. The correct trend is
Li < K < Na < Rb
The basis of long form of periodic table is:- a)Mass number
- b)Atomic weight
- c)Atomic number
- d)Ionic radius
Correct answer is option 'C'. Can you explain this answer?
The basis of long form of periodic table is:
a)
Mass number
b)
Atomic weight
c)
Atomic number
d)
Ionic radius
|
|
Nandini Patel answered |
In long form of periodic table elements are arranged in increasing order of their Atomic number.
Which of the following elements are called representative elements?- a)p block elements only
- b)s and p block elements only
- c)d block elements only
- d)s block elements only
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements are called representative elements?
a)
p block elements only
b)
s and p block elements only
c)
d block elements only
d)
s block elements only
|
|
Gaurav Kumar answered |
The elements of "s" and "p" blocks except "d" group elements are called as representative elements. Their outer shells are not completely filled with electrons. The elements get the nearest inert gas configuration by losing or gaining or sharing of electrons.
Inert gases belong to which block of the periodic table?- a)s-block
- b)d-block
- c)p-block
- d)f-block
Correct answer is 'A'. Can you explain this answer?
Inert gases belong to which block of the periodic table?
a)
s-block
b)
d-block
c)
p-block
d)
f-block
|
|
Rajeev Saxena answered |
The six noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Their atomic numbers are, respectively, 2, 10, 18, 36, 54, and 86.
Which of the following elements belongs to the same group as the element having configuration 1s2 2s2 2p1- a)E: (1s2 2s2 2p6 3s2 3p6 4s2)
- b)(1s2 2s2 2p6 3s2 3p3)
- c)(1s2 2s2 2p6 3s2 3p1)
- d)(1s2 2s2 2p6 3s2 3p5)
Correct answer is option 'C'. Can you explain this answer?
Which of the following elements belongs to the same group as the element having configuration 1s2 2s2 2p1
a)
E: (1s2 2s2 2p6 3s2 3p6 4s2)
b)
(1s2 2s2 2p6 3s2 3p3)
c)
(1s2 2s2 2p6 3s2 3p1)
d)
(1s2 2s2 2p6 3s2 3p5)

|
Sarthika The Hacker Girl answered |
Now see..the first one if u add all the electrons u will get 20 means Ca, similarly the others 15 is P , 13 is Al and 17 is Cl respectively...now the question mentioned configuration 1s^2 2s^2 2p^1 is B( boron)..check the modern periodic table..u will find Al n B is in the same group where the others belong to other group..so the answer is option c..first u take ur chemistry book out and check the elements names, I described ..u will easily at a glance find the answer...Thank You!! Hope I could help you!!:-)
Choose the correct statement:- a)Across a period electropositivity of elements increases
- b)Across a period basic nature of oxides increases
- c)Across a period acidic nature of oxides increases
- d)Across a period metallic character increases
Correct answer is option 'C'. Can you explain this answer?
Choose the correct statement:
a)
Across a period electropositivity of elements increases
b)
Across a period basic nature of oxides increases
c)
Across a period acidic nature of oxides increases
d)
Across a period metallic character increases
|
|
Geetika Shah answered |
The non metallic character decreases down the group.And we know the oxides of non-metal r acidic in genral.Hence acidic character of oxides decreases down d group. Down the group the size of the element increases, electronegativity decreases ,metallic character increases and hence the acidic character decrease.
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:- a)Third element
- b)Fourth element
- c)Second element
- d)First element
Correct answer is option 'D'. Can you explain this answer?
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
a)
Third element
b)
Fourth element
c)
Second element
d)
First element
|
|
Hansa Sharma answered |
According to Newlands' law of octaves when the elements are arranged in order of increasing atomic weights then every eighth element has properties similar to that of the first element.
14 elements after actinium is called
a) Lanthanides
b) Actinides
c) D-block elements
d) P block elements
Correct answer is option 'B'. Can you explain this answer?
|
|
Neha Sharma answered |
The correct answer is Option B.
14 elements after actinium is called Actinides.
Which one of the following configuration represents a metallic character?- a)2, 8, 2
- b)2, 8, 4
- c)2, 8, 7
- d)2, 8, 8
Correct answer is option 'A'. Can you explain this answer?
Which one of the following configuration represents a metallic character?
a)
2, 8, 2
b)
2, 8, 4
c)
2, 8, 7
d)
2, 8, 8
|
|
Pooja Shah answered |
Option ‘a’ represents metallic character of Mg. All others remaining represent nonmetals and metalloids.
Elements of Group-1 are called:- a)Chalcogens
- b)Alkaline earth metals
- c)Alkali metals
- d)Halogens
Correct answer is 'C'. Can you explain this answer?
Elements of Group-1 are called:
a)
Chalcogens
b)
Alkaline earth metals
c)
Alkali metals
d)
Halogens
|
|
Neha Joshi answered |
Elements of group 1, i.e. Li, Na, K, Rb, Cs and Fr, are called alkali metals because they react with water to form alkaline solutions. The pH of solutions usually range between 8-12.
The metallic character of group 14 elements:- a)Decreases and then increases.
- b)Increases from top to bottom in group.
- c)Remains same for all elements of group.
- d)Decreases from top to bottom in group.
Correct answer is option 'B'. Can you explain this answer?
The metallic character of group 14 elements:
a)
Decreases and then increases.
b)
Increases from top to bottom in group.
c)
Remains same for all elements of group.
d)
Decreases from top to bottom in group.
|
|
Preeti Iyer answered |
The metallic character of group 14 elements increases from top to bottom because removal of electron becomes easier on moving down the group. Metallic character increases as you move down an element group in the periodic table. This is because electrons become easier to lose as the atomic radius increases, where there is less attraction between the nucleus and the valence electrons because of the increased distance between them.
Elements of which group are called Halogens?- a)Group 16
- b)Group 17
- c)Group 18
- d)Group 15
Correct answer is option 'B'. Can you explain this answer?
Elements of which group are called Halogens?
a)
Group 16
b)
Group 17
c)
Group 18
d)
Group 15
|
|
Geetika Shah answered |
Group 17 elements are called halogens because halogen is a Greek word which means 'salt producing'. Halogens include fluorine, chlorine, bromine, iodine andastatine. They all are non-metals. They react with metals to form compounds called salts.
Identify the element belonging to third period and 17th group of the periodic table.- a)Sulphur
- b)Silicon
- c)Bromine
- d)Chlorine
Correct answer is option 'D'. Can you explain this answer?
Identify the element belonging to third period and 17th group of the periodic table.
a)
Sulphur
b)
Silicon
c)
Bromine
d)
Chlorine
|
|
Hansa Sharma answered |
There are two elements in the 1st period and eight elements in the 2nd period., The third period starts with the element with Z = 11(sodium). Now, there are nine elements in the third period. Thus, the 3rd period ends with the element with Z = 18(argon) i.e., the element in the 18th group of the third period has Z = 18. Hence, the element in the 17th group of the third period has atomic number Z = 17 ,chlorine(Cl) with atomic mass 35.453, which is a p block element.
Mendeleev's Periodic Table was arranged primarily based on which property of elements?- a)Atomic mass
- b)Electronegativity
- c)Ionization energy
- d)Atomic radius
Correct answer is option 'A'. Can you explain this answer?
Mendeleev's Periodic Table was arranged primarily based on which property of elements?
a)
Atomic mass
b)
Electronegativity
c)
Ionization energy
d)
Atomic radius

|
Lead Academy answered |
Mendeleev's Periodic Table was initially arranged based on increasing atomic mass. He noticed that elements with similar properties appeared at regular intervals when arranged in order of increasing atomic mass.
Chalcogens belong to which group of the periodic table?- a)Group 14
- b)Group 15
- c)Group 16
- d)Group 17
Correct answer is option 'C'. Can you explain this answer?
Chalcogens belong to which group of the periodic table?
a)
Group 14
b)
Group 15
c)
Group 16
d)
Group 17

|
Infinity Academy answered |
The Chalcogens are the elements found in Group 16 of the periodic table. The name "chalcogen" comes from the Greek words chalcos (meaning "ore") and gen (meaning "to form"), because many ores contain oxygen and sulfur, two of the chalcogens.
The elements in Group 16 include:
- Oxygen (O)
- Sulfur (S)
- Selenium (Se)
- Tellurium (Te)
- Polonium (Po)
These elements share some chemical properties, such as forming compounds by gaining two electrons, leading to a common oxidation state of -2. Oxygen, the most well-known chalcogen, is a vital component of water and air, while sulfur is widely used in industries like rubber manufacturing and fertilizers.
The order of Decreasing radius isa)Van der Waals’radius > Metallic radius > Covalent radiusb)Covalent radius Van der Waals’radiusc)Metallic radius > Van der Waals’radius > Covalent radiusd)Van der Waals’radius > Covalent radius > Metallic radiusCorrect answer is option 'A'. Can you explain this answer?

|
Raghav Chakraborty answered |
vanderwaal radius > Metallic radius > Covalent radius.
The modern periodic table is divided into how many blocks?- a)3
- b)2
- c)1
- d)4
Correct answer is option 'D'. Can you explain this answer?
The modern periodic table is divided into how many blocks?
a)
3
b)
2
c)
1
d)
4
|
|
Shreya Gupta answered |
The periodic table is divided into four blocks that are called s, p, d, and f. Here is an explanation of each block: The s block makes up the first two columns of the periodic table. The elements in this block are all metals, and they react when water is present.
Metalloids show the properties of- a)properties that are characteristic of both metals and viscous liquids
- b)properties that are characteristic of both metals and plastics
- c)properties that are characteristic of both metals and nonmetals
- d)properties that are characteristic of both metals and gases
Correct answer is option 'C'. Can you explain this answer?
Metalloids show the properties of
a)
properties that are characteristic of both metals and viscous liquids
b)
properties that are characteristic of both metals and plastics
c)
properties that are characteristic of both metals and nonmetals
d)
properties that are characteristic of both metals and gases

|
Janhavi Banerjee answered |
Metalloids shows both the properties of metals as well as non metals.
Which of the following will have the most negative electron gain enthalpy and which one the least negative? P, S, Cl, F.- a)Cl, P
- b)P,Cl
- c)P,S
- d)none
Correct answer is option 'A'. Can you explain this answer?
Which of the following will have the most negative electron gain enthalpy and which one the least negative? P, S, Cl, F.
a)
Cl, P
b)
P,Cl
c)
P,S
d)
none
|
|
Sinjini Pillai answered |
**Answer:**
The electron gain enthalpy is the energy released when an atom gains an electron to form a negative ion. It is a measure of the tendency of an atom to accept an electron.
To determine which element will have the most negative electron gain enthalpy and which one will have the least negative, we need to consider the factors that influence electron gain enthalpy.
1. **Nuclear charge**: The greater the nuclear charge, the stronger the attraction for the incoming electron, resulting in a more negative electron gain enthalpy.
2. **Atomic size**: The smaller the atomic size, the closer the valence electrons are to the nucleus, resulting in a stronger attraction for the incoming electron and a more negative electron gain enthalpy.
Considering these factors, we can analyze the elements given in the options:
a) Cl, P: Chlorine (Cl) has a higher nuclear charge than Phosphorus (P), so it will have a greater attraction for the incoming electron. Additionally, chlorine is smaller in size compared to phosphorus. Therefore, chlorine will have the most negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
b) P, Cl: As mentioned above, chlorine (Cl) has a higher nuclear charge and is smaller in size compared to phosphorus (P). Therefore, chlorine will have a more negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
c) P, S: Sulfur (S) has a higher nuclear charge and is smaller in size compared to phosphorus (P). Therefore, sulfur will have a more negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
d) None: The answer cannot be "none" since we have already determined that chlorine will have the most negative electron gain enthalpy.
In conclusion, the correct answer is option 'A': Cl will have the most negative electron gain enthalpy, and P will have the least negative electron gain enthalpy.
The electron gain enthalpy is the energy released when an atom gains an electron to form a negative ion. It is a measure of the tendency of an atom to accept an electron.
To determine which element will have the most negative electron gain enthalpy and which one will have the least negative, we need to consider the factors that influence electron gain enthalpy.
1. **Nuclear charge**: The greater the nuclear charge, the stronger the attraction for the incoming electron, resulting in a more negative electron gain enthalpy.
2. **Atomic size**: The smaller the atomic size, the closer the valence electrons are to the nucleus, resulting in a stronger attraction for the incoming electron and a more negative electron gain enthalpy.
Considering these factors, we can analyze the elements given in the options:
a) Cl, P: Chlorine (Cl) has a higher nuclear charge than Phosphorus (P), so it will have a greater attraction for the incoming electron. Additionally, chlorine is smaller in size compared to phosphorus. Therefore, chlorine will have the most negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
b) P, Cl: As mentioned above, chlorine (Cl) has a higher nuclear charge and is smaller in size compared to phosphorus (P). Therefore, chlorine will have a more negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
c) P, S: Sulfur (S) has a higher nuclear charge and is smaller in size compared to phosphorus (P). Therefore, sulfur will have a more negative electron gain enthalpy, and phosphorus will have a less negative electron gain enthalpy.
d) None: The answer cannot be "none" since we have already determined that chlorine will have the most negative electron gain enthalpy.
In conclusion, the correct answer is option 'A': Cl will have the most negative electron gain enthalpy, and P will have the least negative electron gain enthalpy.
Which of the following statements is false?- a)Alkali metals form covalent bonds with oxygen.
- b)Alkali metals have relatively low first ionization energies
- c)Alkali metals forms oxides that act as basic anhydrides.
- d)Alkali metals usually have a +1 oxidation state
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is false?
a)
Alkali metals form covalent bonds with oxygen.
b)
Alkali metals have relatively low first ionization energies
c)
Alkali metals forms oxides that act as basic anhydrides.
d)
Alkali metals usually have a +1 oxidation state
|
|
Pooja Mehta answered |
Lithium, sodium and potassium all react vigorously with water. Hydrogen gas bubbles off and, in the case of potassium, this burns with a lilac flame. The metal hydroxide is formed which is a strong alkali. Alkali metals need to be stored under oil to prevent them reacting with the oxygen and water vapour in the air.
Alkaline earth metals include:- a)Group 18 elements
- b)Group 2 elements
- c)Group 1 element
- d)Group 17 elements
Correct answer is option 'B'. Can you explain this answer?
Alkaline earth metals include:
a)
Group 18 elements
b)
Group 2 elements
c)
Group 1 element
d)
Group 17 elements
|
|
Hansa Sharma answered |
Group 1-Alkali metal,
group2- Alkali earth metals
group2- Alkali earth metals
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order? (At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)- a)Fe > Mn > Co > Cr
- b)Mn > Fe > Cr > Co
- c)Cr > Mn > Co > Fe
- d)Co > Mn > Fe > Cr
Correct answer is option 'B'. Can you explain this answer?
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order? (At. nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
a)
Fe > Mn > Co > Cr
b)
Mn > Fe > Cr > Co
c)
Cr > Mn > Co > Fe
d)
Co > Mn > Fe > Cr

|
Gaurav Saini answered |
Mn has most stable +2 stable because Mn+2 had d5 configuration which is most stable.
The sequence of ionic mobility in aqueous solution is - a)K+ > Na+ > Rb+ > Cs+
- b)Cs+ > Rb+ > K+ >Na+
- c)Rb+ > K+> Cs+ > Na+
- d)Na+ > K+ >Rb+ >Cs+
Correct answer is option 'B'. Can you explain this answer?
The sequence of ionic mobility in aqueous solution is
a)
K+ > Na+ > Rb+ > Cs+
b)
Cs+ > Rb+ > K+ >Na+
c)
Rb+ > K+> Cs+ > Na+
d)
Na+ > K+ >Rb+ >Cs+
|
|
Hansa Sharma answered |
Smaller the size of cation, higher will be the hydration and its effective size will increase and hence mobility in aqueous solution will decrease. Larger size ions have more ionic mobility due to less hydration. Thus the degree of hydration of M+ ions decreases from Li+ to Cs+. Consequently the radii of the hydrated ion decreases from Li+ to Cs+. Hence the ionic conductance of these hydrated ions increases from Li+ to Cs+
In Dobereiner's Triads, elements were grouped based on their similar chemical properties. Which of the following elements was not part of any known Dobereiner's Triad?- a)Lithium
- b)Sodium
- c)Carbon
- d)Chlorine
Correct answer is option 'C'. Can you explain this answer?
In Dobereiner's Triads, elements were grouped based on their similar chemical properties. Which of the following elements was not part of any known Dobereiner's Triad?
a)
Lithium
b)
Sodium
c)
Carbon
d)
Chlorine

|
EduRev NEET answered |
Dobereiner's Triads were groups of three elements with similar chemical properties, where the atomic mass of the middle element was approximately equal to the average of the other two. Carbon, being a nonmetal with a unique set of properties, did not fit into any known triad.
According to Moseley, a straight-line graph is obtained on plotting-- a)The frequencies of characteristic X-rays of elements against their atomic numbers.
- b)The square of the frequencies of characteristic X-rays of elements against their atomic numbers
- c)The square root of the frequencies of characteristic X-rays of elements against their atomic numbers
- d)The reciprocal of the frequencies of characteristic X-rays of elements against their atomic numbers.
Correct answer is option 'C'. Can you explain this answer?
According to Moseley, a straight-line graph is obtained on plotting-
a)
The frequencies of characteristic X-rays of elements against their atomic numbers.
b)
The square of the frequencies of characteristic X-rays of elements against their atomic numbers
c)
The square root of the frequencies of characteristic X-rays of elements against their atomic numbers
d)
The reciprocal of the frequencies of characteristic X-rays of elements against their atomic numbers.
|
|
Ishita Mukherjee answered |
**Explanation:**
**Moseley's Law:**
Moseley's Law states that the square root of the frequency of characteristic X-rays emitted by an element is directly proportional to the atomic number of the element.
**Plotting the Frequencies of Characteristic X-rays:**
In order to understand Moseley's Law and obtain a straight-line graph, we need to plot the frequencies of characteristic X-rays of elements against their atomic numbers. This means we will have the frequency (f) on the y-axis and the atomic number (Z) on the x-axis.
**Interpreting the Options:**
a) The frequencies of characteristic X-rays of elements against their atomic numbers: This option is correct because it represents the correct plot required to obtain a straight-line graph according to Moseley's Law.
b) The square of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because squaring the frequencies would not result in a straight-line graph. The relationship between the square of the frequency and atomic number is not linear.
c) The square root of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because taking the square root of the frequencies would not result in a straight-line graph. The relationship between the square root of the frequency and atomic number is not linear.
d) The reciprocal of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because taking the reciprocal of the frequencies would not result in a straight-line graph. The relationship between the reciprocal of the frequency and atomic number is not linear.
**Conclusion:**
Based on Moseley's Law, the correct option is a) The frequencies of characteristic X-rays of elements against their atomic numbers. This plot will result in a straight-line graph, demonstrating the linear relationship between the frequency of characteristic X-rays and the atomic number of elements.
**Moseley's Law:**
Moseley's Law states that the square root of the frequency of characteristic X-rays emitted by an element is directly proportional to the atomic number of the element.
**Plotting the Frequencies of Characteristic X-rays:**
In order to understand Moseley's Law and obtain a straight-line graph, we need to plot the frequencies of characteristic X-rays of elements against their atomic numbers. This means we will have the frequency (f) on the y-axis and the atomic number (Z) on the x-axis.
**Interpreting the Options:**
a) The frequencies of characteristic X-rays of elements against their atomic numbers: This option is correct because it represents the correct plot required to obtain a straight-line graph according to Moseley's Law.
b) The square of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because squaring the frequencies would not result in a straight-line graph. The relationship between the square of the frequency and atomic number is not linear.
c) The square root of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because taking the square root of the frequencies would not result in a straight-line graph. The relationship between the square root of the frequency and atomic number is not linear.
d) The reciprocal of the frequencies of characteristic X-rays of elements against their atomic numbers: This option is incorrect because taking the reciprocal of the frequencies would not result in a straight-line graph. The relationship between the reciprocal of the frequency and atomic number is not linear.
**Conclusion:**
Based on Moseley's Law, the correct option is a) The frequencies of characteristic X-rays of elements against their atomic numbers. This plot will result in a straight-line graph, demonstrating the linear relationship between the frequency of characteristic X-rays and the atomic number of elements.
At present how many elements are known?- a)110
- b)118
- c)63
- d)105
Correct answer is option 'B'. Can you explain this answer?
At present how many elements are known?
a)
110
b)
118
c)
63
d)
105

|
Stepway Academy answered |
- There are 118 elements on the periodic table, Four with atomic numbers – 113 (Nihonium), 115 (Moskovi), 117 (Tennesin) and 118 (Oganesson) – were added in 2016.
- With the discoveries of new elements, it's difficult to ascertain how long the table is going to be in the future.
One of the following options is not used for explaining atomic radius- a)coordinate radius
- b)covalent radius
- c)van der waals’radius
- d)metallic radius
Correct answer is option 'A'. Can you explain this answer?
One of the following options is not used for explaining atomic radius
a)
coordinate radius
b)
covalent radius
c)
van der waals’radius
d)
metallic radius
|
|
Pooja Mehta answered |
Atomic radius
The distance from the centre of the nucleus to the outermost shell containing electrons.
or
The distance from the centre of the nucleus to the point up to which the density of the electron cloud is maximum.
Types of atomic radii
1) Covalent radius
2) Van der waals radius
3)Metallic radius
The p-block elements constitute elements belonging to which groups?- a)Group 13 to 18
- b)Group 1 to 18
- c)Group 1 to 13
- d)Group 10 to 14
Correct answer is option 'A'. Can you explain this answer?
The p-block elements constitute elements belonging to which groups?
a)
Group 13 to 18
b)
Group 1 to 18
c)
Group 1 to 13
d)
Group 10 to 14

|
Nishanth Verma answered |
The p-block elements constitute elements belonging to group 13 to 18 and these together with the s-Block elements are called Representative elements.
Newland's Law of Octaves suggested that elements exhibited similar properties at regular intervals. However, this law failed to accommodate the discovery of:- a)Noble gases
- b)Halogens
- c)Transition metals
- d)Lanthanides and Actinides
Correct answer is option 'A'. Can you explain this answer?
Newland's Law of Octaves suggested that elements exhibited similar properties at regular intervals. However, this law failed to accommodate the discovery of:
a)
Noble gases
b)
Halogens
c)
Transition metals
d)
Lanthanides and Actinides
|
|
Ishita Mukherjee answered |
Explanation:
Newlands Law of Octaves
- Proposed by John Newlands in 1864
- Suggested that elements exhibited similar properties at regular intervals of atomic weight, with every eighth element having similar properties
Failure to accommodate the discovery of Noble Gases
- Noble gases were discovered after the formulation of Newlands Law of Octaves
- Noble gases such as helium, neon, argon, krypton, xenon, and radon do not fit into the pattern of octaves as they have unique properties and do not exhibit similarities with the elements in the same period
Reasons for the failure
- Noble gases have a stable electronic configuration with a full outer shell, making them chemically inert
- Their properties are distinct from other elements, making it difficult to fit them into the periodic table based on Newlands' law
Conclusion
- The discovery of noble gases posed a challenge to Newlands Law of Octaves as it did not account for elements with unique properties and stable configurations
- This led to the further development and refinement of the periodic table, eventually leading to the modern periodic table based on atomic number and electronic configuration.
Newlands Law of Octaves
- Proposed by John Newlands in 1864
- Suggested that elements exhibited similar properties at regular intervals of atomic weight, with every eighth element having similar properties
Failure to accommodate the discovery of Noble Gases
- Noble gases were discovered after the formulation of Newlands Law of Octaves
- Noble gases such as helium, neon, argon, krypton, xenon, and radon do not fit into the pattern of octaves as they have unique properties and do not exhibit similarities with the elements in the same period
Reasons for the failure
- Noble gases have a stable electronic configuration with a full outer shell, making them chemically inert
- Their properties are distinct from other elements, making it difficult to fit them into the periodic table based on Newlands' law
Conclusion
- The discovery of noble gases posed a challenge to Newlands Law of Octaves as it did not account for elements with unique properties and stable configurations
- This led to the further development and refinement of the periodic table, eventually leading to the modern periodic table based on atomic number and electronic configuration.
The elements charecterised by the filling of 4 f-orbitals, are:- a)Alkali metals
- b)Lanthanoids
- c)Alkaline earth metals
- d)Transition elements)
Correct answer is option 'B'. Can you explain this answer?
The elements charecterised by the filling of 4 f-orbitals, are:
a)
Alkali metals
b)
Lanthanoids
c)
Alkaline earth metals
d)
Transition elements)
|
|
Rohan Singh answered |
The f block elements are the lanthanides and actinides and are called the inner transition elements because of their placement in the periodic table due to their electron configurations. The f orbitals of the electron shell are filled with “n-2.” There is a maximum of fourteen electrons that can occupy the f orbitals.
Chapter doubts & questions for Classification of Elements and Periodicity in Properties - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Classification of Elements and Periodicity in Properties - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup