All Exams >
JAMB >
Physics for JAMB >
All Questions
All questions of Quantity of Heat & Change of State for JAMB Exam
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:- a)2°C
- b)0°C
- c)5°C
- d)2.5°C
Correct answer is option 'B'. Can you explain this answer?
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:
a)
2°C
b)
0°C
c)
5°C
d)
2.5°C
|
|
Riya Banerjee answered |
Heat absorbed by 5g ice when it converted to at 0° C = 5 x 80 = 400 cal.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)- a)5R/2
- b)3 R
- c)2R
- d)5 R
Correct answer is option 'D'. Can you explain this answer?
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)
a)
5R/2
b)
3 R
c)
2R
d)
5 R
|
|
Hansa Sharma answered |
At constant pressure
dQ= nCpdT
=1*(5R/2)*2
=5R
dQ= nCpdT
=1*(5R/2)*2
=5R
Can you explain the answer of this question below:Water is used as coolant in automobiles radiators because
- A:
it has high specific heat capacity
- B:
it is easily available
- C:
it is easy to carry
- D:
it is cheap
The answer is a.
Water is used as coolant in automobiles radiators because
it has high specific heat capacity
it is easily available
it is easy to carry
it is cheap
|
|
Lavanya Menon answered |
Water is used as a coolant in automobiles radiators because it has high specific heat capacity. So, it absorbs a large amount of heat for a degree rise in temperature.
The temperature range upto which Newton’s law of cooling holds good- a)30-35°C
- b)25-30°C
- c)30-40°C
- d)20-25°C
Correct answer is option 'D'. Can you explain this answer?
The temperature range upto which Newton’s law of cooling holds good
a)
30-35°C
b)
25-30°C
c)
30-40°C
d)
20-25°C
|
|
Hansa Sharma answered |
Newton’s law of cooling is to be used at temperatures around room temperature.
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is- a)(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
- b)(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
- c)(t1 + t2 +t3 )/3
- d)3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
Correct answer is option 'B'. Can you explain this answer?
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is
a)
(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
b)
(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
c)
(t1 + t2 +t3 )/3
d)
3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
|
|
Raghav Bansal answered |
For the composite system, energy conservation yields no net energy flow in or out of the system.
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
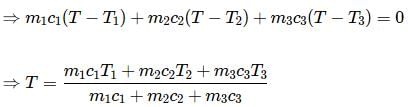
Here in the question m1=m2=m3
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
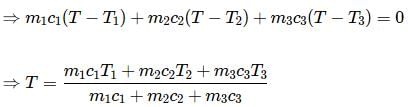
Here in the question m1=m2=m3
The heat of sun comes to us through:- a)convection
- b)conduction
- c)Sublimation
- d)Radiation
Correct answer is option 'D'. Can you explain this answer?
The heat of sun comes to us through:
a)
convection
b)
conduction
c)
Sublimation
d)
Radiation
|
|
Om Desai answered |
The sun heats the earth through radiation. Since there is no medium (like the gas in our atmosphere) in space, radiation is the primary way that heat travels in space. When the heat reaches the earth it warms the molecules of the atmosphere, and they warm other molecules and so on.
The process by which heat is transferred from one place to another without heating the intervening medium is known as- a)convection
- b)radiation
- c)sublimation
- d)fusion
Correct answer is option 'B'. Can you explain this answer?
The process by which heat is transferred from one place to another without heating the intervening medium is known as
a)
convection
b)
radiation
c)
sublimation
d)
fusion

|
Pioneer Academy answered |
Radiation alludes to the mechanism in which heat is transmitted without any physical contact between objects. It uses electromagnetic waves for transfer of heat.
Melting point of ice decreases with- a)Decrease in pressure
- b)Increase in temperature
- c)Decrease in temperature
- d)Increase in pressure
Correct answer is option 'D'. Can you explain this answer?
Melting point of ice decreases with
a)
Decrease in pressure
b)
Increase in temperature
c)
Decrease in temperature
d)
Increase in pressure
|
|
Preeti Iyer answered |
When ice melts the volume decreases and an increase in pressure will support this phenomena according to Le Chatalier’s rule.
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.- a)500°C
- b)900°C
- c)953.6∘C
- d)706.80 °C
Correct answer is option 'C'. Can you explain this answer?
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.
a)
500°C
b)
900°C
c)
953.6∘C
d)
706.80 °C
|
|
Gaurav Kumar answered |
Mass of Iron = 100g
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Total heat gained = Total heat lost.
So,100/1000× 470 × (θ – 60) = 250/1000 × 4200 × (60 – 20)
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
What is the expression for temperature gradient?- a)
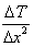
- b)
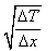
- c)
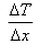
- d)
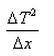
Correct answer is option 'C'. Can you explain this answer?
What is the expression for temperature gradient?
a)
b)
c)
d)
|
|
Krishna Iyer answered |
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location.
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
According to law of calorimetry, which of the given relation is true?- a)Heat gained ≥ Heat lost
- b)Heat gained = Heat lost
- c)Heat gained > Heat lost
- d)Heat lost > Heat gained
Correct answer is option 'B'. Can you explain this answer?
According to law of calorimetry, which of the given relation is true?
a)
Heat gained ≥ Heat lost
b)
Heat gained = Heat lost
c)
Heat gained > Heat lost
d)
Heat lost > Heat gained
|
|
Anjali Iyer answered |
A principle of calorimetry states that if there is no loss of heat in surrounding the total heat lost by hot body equal to the total heat gained by a cold body.
i.e. heat loss = heat gain
A device in which heat measurement can be made is called- a)Joule meter
- b)Calorimeter
- c)Thermal meter
- d)Gauge meter
Correct answer is option 'B'. Can you explain this answer?
A device in which heat measurement can be made is called
a)
Joule meter
b)
Calorimeter
c)
Thermal meter
d)
Gauge meter
|
|
Rajesh Gupta answered |
A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.- a)temperature of the surroundings
- b)excess temperature of the body over the surroundings
- c)temperature of the body
- d)temperature of the body + temperature of the surroundings
Correct answer is option 'B'. Can you explain this answer?
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.
a)
temperature of the surroundings
b)
excess temperature of the body over the surroundings
c)
temperature of the body
d)
temperature of the body + temperature of the surroundings
|
|
Geetika Shah answered |
Newton's law of cooling states that the heat released by a body with respect to time (or) the rate of heat released is directly proportional to the difference between the body's temperature and the surrounding temperature.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
Skating is possible on snow due to the formation of water below the skates. This water below skates comes as result of- a)Increase in temperature between skates and snow
- b)Increase in pressure between skates and snow
- c)Force due to weight of the skater
- d)Decrease in pressure between skates and snow
Correct answer is option 'B'. Can you explain this answer?
Skating is possible on snow due to the formation of water below the skates. This water below skates comes as result of
a)
Increase in temperature between skates and snow
b)
Increase in pressure between skates and snow
c)
Force due to weight of the skater
d)
Decrease in pressure between skates and snow
|
|
Naina Sharma answered |
As the pressure increases between the skates and ice, the ice starts melting below 0o C.
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?- a)temperature of black body should be less than zero
- b)temperature of black body should be more than zero
- c)temperature of black body should be equal to zero
- d)all of the above
Correct answer is option 'B'. Can you explain this answer?
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?
a)
temperature of black body should be less than zero
b)
temperature of black body should be more than zero
c)
temperature of black body should be equal to zero
d)
all of the above
|
|
Suresh Reddy answered |
Temperature of black body should be more than zero. Obvious answer. If the temperature is not greater than 0 then there will be no radiation.
What is the coefficient of thermal conductivity K?- a)ΔQt
- b)
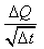
- c)
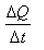
- d)
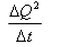
Correct answer is option 'C'. Can you explain this answer?
What is the coefficient of thermal conductivity K?
a)
ΔQt
b)
c)
d)

|
Ciel Knowledge answered |
K is rate of change of charge.
Mercury is a liquid which is heated by:- a)Convection
- b)Radiation
- c)Conduction
- d)transfusion
Correct answer is option 'C'. Can you explain this answer?
Mercury is a liquid which is heated by:
a)
Convection
b)
Radiation
c)
Conduction
d)
transfusion
|
|
Geetika Shah answered |
Hg expands on heat (thermometers) it acts as a good conducting metal.
The temperature and pressure at which all three phases of a substances coexist is called- a)Fusion point
- b)Triple point
- c)Sublimation point
- d)Melting Point
Correct answer is option 'B'. Can you explain this answer?
The temperature and pressure at which all three phases of a substances coexist is called
a)
Fusion point
b)
Triple point
c)
Sublimation point
d)
Melting Point
|
|
Rajeev Saxena answered |
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet.
Value of coefficient of thermal coefficient is:- a)same incase of conductors and insulators
- b)Good incase of conductors and small incase of insulators
- c)Does not depend on insulators and conductors
- d)Small incase of insulators and good incase of insulators
Correct answer is option 'B'. Can you explain this answer?
Value of coefficient of thermal coefficient is:
a)
same incase of conductors and insulators
b)
Good incase of conductors and small incase of insulators
c)
Does not depend on insulators and conductors
d)
Small incase of insulators and good incase of insulators
|
|
Raghav Bansal answered |
Conductors have free electrons in them whereas insulators don’t have any. Therefore, conductors conduct heat and electricity better than insulators. Therefore the value of thermal coefficient is good in case of conductors and less in case in insulators.
Which of the given phenomenon is not related to convection?- a)In winter metallic handles appear colder than wooden door
- b)Maintaining comfortable room temperature in cold countries
- c)Formation of trade winds
- d)Formation of land and sea breezes
Correct answer is option 'A'. Can you explain this answer?
Which of the given phenomenon is not related to convection?
a)
In winter metallic handles appear colder than wooden door
b)
Maintaining comfortable room temperature in cold countries
c)
Formation of trade winds
d)
Formation of land and sea breezes

|
Harika Chittiboyina answered |
Convection is the movement of surrounding medium according to density by which transfer of heat takes place. Whereas in option A it tells about the transfer of heat due to molecules inside conductor which is conduction
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as- a)conduction
- b)convection
- c)Sublimation
- d)Fusion
Correct answer is option 'A'. Can you explain this answer?
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as
a)
conduction
b)
convection
c)
Sublimation
d)
Fusion
|
|
Geetika Shah answered |
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as conduction. This is the definition of conduction.
An increase in temperature in a liquid would cause a phase change to which of the following?- a)Gas
- b)liquid
- c)solid
- d)plasma
Correct answer is option 'A'. Can you explain this answer?
An increase in temperature in a liquid would cause a phase change to which of the following?
a)
Gas
b)
liquid
c)
solid
d)
plasma
|
|
Neha Sharma answered |
Increasing the temperature of liquid, increases the average K.E. of the molecules. The molecules start moving vigorously in all the directions, thereby increasing and the inter-molecular space between them. Thus, the liquid changes into gas.
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called- a)Sublimation
- b)radiaton
- c)Conduction
- d)Convection
Correct answer is option 'D'. Can you explain this answer?
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called
a)
Sublimation
b)
radiaton
c)
Conduction
d)
Convection
|
|
Rajat Patel answered |
In this process, heat is transferred in the liquid and gases from a region of higher temperature to a region of lower temperature. Convection heat transfer occurs partly due to the actual movement of molecules or due to the mass transfer.
SI unit of thermal conductivity is- a)Js K
- b)Js-1 K
- c)Js K-1
- d)Jm-1s-1 K-1
Correct answer is option 'D'. Can you explain this answer?
SI unit of thermal conductivity is
a)
Js K
b)
Js-1 K
c)
Js K-1
d)
Jm-1s-1 K-1
|
|
Pooja Shah answered |
K = power × thickness (m) ÷ area × ∆T
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to- a)Temperature of two bodies
- b)Nature of two bodies
- c)Pressure difference of the body
- d)Size of the two bodies
Correct answer is option 'A'. Can you explain this answer?
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to
a)
Temperature of two bodies
b)
Nature of two bodies
c)
Pressure difference of the body
d)
Size of the two bodies

|
Mahendra Pal Sunda answered |
Where are the two bodies and rate of loss of heat also depends on sigma( property of material)
A student added a drop of red food coloring to 4 beakers of water.Each beaker contained 100 ml of a different temperature water. The student recorded how long it took each beaker to mix completely (without stirring). The following table shows her results:
 What conclusion should the student make from this experiment? Particles are moving
What conclusion should the student make from this experiment? Particles are moving - a)faster in warm water so they mix faster
- b)slowly in warm water so they mix faster
- c)faster in warm water so they mix slowly
- d)slowly in warm water so they mix slowly
Correct answer is option 'A'. Can you explain this answer?
A student added a drop of red food coloring to 4 beakers of water.Each beaker contained 100 ml of a different temperature water. The student recorded how long it took each beaker to mix completely (without stirring). The following table shows her results:


What conclusion should the student make from this experiment? Particles are moving
a)
faster in warm water so they mix faster
b)
slowly in warm water so they mix faster
c)
faster in warm water so they mix slowly
d)
slowly in warm water so they mix slowly
|
|
Preeti Iyer answered |
Adding heat energy to water increases the motion of the water molecules and the molecules of food coloring. The faster moving molecules cause the food coloring to mix into the water faster. The water molecules and food coloring molecules in cold water don't move as quickly as in hot water.
SI unit of latent heat is- a)Cal/ Kg
- b)J Kg
- c)J Kg -2
- d)J/Kg
Correct answer is option 'D'. Can you explain this answer?
SI unit of latent heat is
a)
Cal/ Kg
b)
J Kg
c)
J Kg -2
d)
J/Kg
|
|
Jyoti Dey answered |
The correct answer is option 'D', J/Kg.
Latent heat is the amount of heat energy required or released during a phase change of a substance, such as melting or vaporization, at a constant temperature and pressure. It is a specific type of heat energy associated with the change in the internal energy of a substance, without a change in its temperature.
There are two types of latent heat: latent heat of fusion and latent heat of vaporization. The latent heat of fusion refers to the heat energy required to change a substance from a solid to a liquid state, while the latent heat of vaporization refers to the heat energy required to change a substance from a liquid to a gaseous state.
The SI unit of latent heat is Joule per kilogram (J/Kg). The Joule is the SI unit of energy, and the kilogram is the SI unit of mass. So, when we express the latent heat in the SI unit, we are essentially referring to the amount of energy required or released per unit mass of the substance during a phase change.
The SI unit of latent heat can be derived by considering the equation Q = mL, where Q is the heat energy required or released, m is the mass of the substance, and L is the latent heat. By rearranging the equation, we get L = Q/m. Since Q is in Joules and m is in kilograms, the SI unit of latent heat is J/Kg.
So, option 'D', J/Kg, is the correct answer for the SI unit of latent heat.
Latent heat is the amount of heat energy required or released during a phase change of a substance, such as melting or vaporization, at a constant temperature and pressure. It is a specific type of heat energy associated with the change in the internal energy of a substance, without a change in its temperature.
There are two types of latent heat: latent heat of fusion and latent heat of vaporization. The latent heat of fusion refers to the heat energy required to change a substance from a solid to a liquid state, while the latent heat of vaporization refers to the heat energy required to change a substance from a liquid to a gaseous state.
The SI unit of latent heat is Joule per kilogram (J/Kg). The Joule is the SI unit of energy, and the kilogram is the SI unit of mass. So, when we express the latent heat in the SI unit, we are essentially referring to the amount of energy required or released per unit mass of the substance during a phase change.
The SI unit of latent heat can be derived by considering the equation Q = mL, where Q is the heat energy required or released, m is the mass of the substance, and L is the latent heat. By rearranging the equation, we get L = Q/m. Since Q is in Joules and m is in kilograms, the SI unit of latent heat is J/Kg.
So, option 'D', J/Kg, is the correct answer for the SI unit of latent heat.
Among the following methods of heat transfer, gravity does not play any part in- a)Convection
- b)Radiation and conduction
- c)radiation
- d)convection and conduction
Correct answer is option 'B'. Can you explain this answer?
Among the following methods of heat transfer, gravity does not play any part in
a)
Convection
b)
Radiation and conduction
c)
radiation
d)
convection and conduction
|
|
Rajat Patel answered |
Gravity does not play any part in radiation and conduction because in both these processes heat is transferred without any motion of the medium particles.
What happens to the volume of the substance when the temperature increases?- a)decreases
- b)increases
- c)remains same
- d)not measurable
Correct answer is option 'B'. Can you explain this answer?
What happens to the volume of the substance when the temperature increases?
a)
decreases
b)
increases
c)
remains same
d)
not measurable
|
|
Rajat Patel answered |
In general, liquids tend to get “thinner” when their temperature increases. In general, the liquids tend to expand when their temperature increases. For example, the same mass of boiling water occupies more volume at 100 degrees Celsius than at 20 degrees Celsius. Therefore, increasing temperature decreases density.
Which diagram is the best depiction of the direction of flow of diffusion?- a)
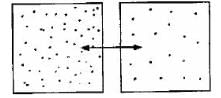
- b)
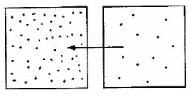
- c)
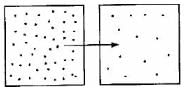
- d)none of these
Correct answer is option 'C'. Can you explain this answer?
Which diagram is the best depiction of the direction of flow of diffusion?
a)

b)

c)

d)
none of these
|
|
Gaurav Kumar answered |
The net flow of solute is from its higher concentration to lower concentration.
Which is the fastest mode of heat transfer?- a)convection
- b)conduction
- c)Transition
- d)Radiation
Correct answer is option 'D'. Can you explain this answer?
Which is the fastest mode of heat transfer?
a)
convection
b)
conduction
c)
Transition
d)
Radiation
|
|
Arun Khanna answered |
Answer: Radiation is the fastest mode of transfer of heat, because radiation travels at the speed of light, which is very quick. The slowest mode of transfer of heat is conduction because it takes place from particle to particle.
What are units of K?- a)Wm-2K-1
- b)WmK
- c)Wm-1K-1
- d)Wm-1K-2
Correct answer is option 'C'. Can you explain this answer?
What are units of K?
a)
Wm-2K-1
b)
WmK
c)
Wm-1K-1
d)
Wm-1K-2
|
|
Arun Khanna answered |
So for a first order reaction, so for first order, a first order reaction rate law is rate is equal to our rate constant k times the concentration of our reactant raised to the first power. Units of rate are molar per second, and the units of concentration are always going to be molar.
A rigid container of volume 0.5 m³ contains 1.0 kg of water at 120°C (vf = 0.00106 m³/kg, vg = 0.8908 m³/kg). The state of water is- a)Compressed liquid
- b)Saturated liquid
- c)Superheated vapor
- d)A mixture of saturated liquid and saturated vapor
Correct answer is option 'D'. Can you explain this answer?
A rigid container of volume 0.5 m³ contains 1.0 kg of water at 120°C (vf = 0.00106 m³/kg, vg = 0.8908 m³/kg). The state of water is
a)
Compressed liquid
b)
Saturated liquid
c)
Superheated vapor
d)
A mixture of saturated liquid and saturated vapor

|
Ambition Institute answered |
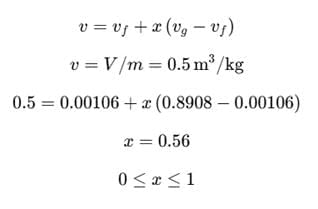
x = 0, Saturated liquid
x = 1, Saturated vapor
x = 1, Saturated vapor
As x value lies between 0 and 1, therefore it is a mixture of saturated liquid and saturated vapor.
Which of the given relation is true for Newton’s law of cooling?- a)
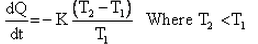
- b)
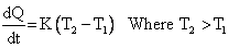
- c)
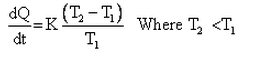
- d)
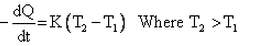
Correct answer is option 'D'. Can you explain this answer?
Which of the given relation is true for Newton’s law of cooling?
a)
b)
c)
d)
|
|
Arka Bose answered |
Newton's law of cooling states that the heat released by a body with respect to time (or) the rate of heat released is directly proportional to the difference between the body's temperature and the surrounding temperature.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
Chapter doubts & questions for Quantity of Heat & Change of State - Physics for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Quantity of Heat & Change of State - Physics for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Physics for JAMB
259 videos|253 docs|230 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup