All Exams >
EmSAT Achieve >
Physics for EmSAT Achieve >
All Questions
All questions of Matter Waves: De Broglie Wavelength for EmSAT Achieve Exam
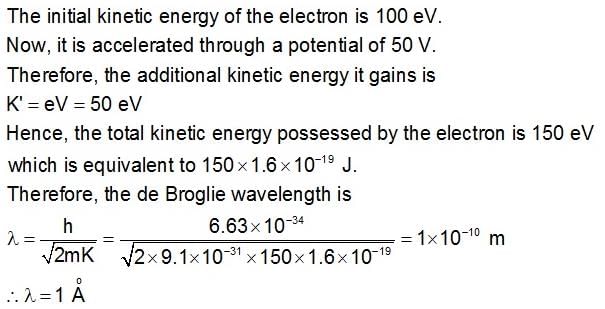
What is the de Broglie wavelength associated with ... morean electron, accelerated through a potential difference of 100 volts?a)12.3 nmb)0.127 nmc)123 nmd)1.23 nmCorrect answer is option 'B'. Can you explain this answer?
|
|
Geetika Shah answered |

and as we know 1Å = 0.1 nm, so 1.227Å = 0.122 nm
Number of ejected photoelectrons increases with increase- a)never
- b)in frequency of light
- c)in wavelength of light
- d)in intensity of light
Correct answer is option 'D'. Can you explain this answer?
Number of ejected photoelectrons increases with increase
a)
never
b)
in frequency of light
c)
in wavelength of light
d)
in intensity of light
|
|
Rohan Singh answered |
A photon is the smallest possible quantum of light. In general when you turn up the intensity of light you are increasing the number of photons per second that are emitted by the light source. Therefore the intensity of the light can indeed be changed independently of the frequency (or color) of the light.
Photons with energy 5eV are incident on a cathode C, on a photoelectric cell. The maximum energy of the emitted photoelectrons is 2eV. When photons of energy 6eV are incident on C, no photoelectrons will reach the anode A if the stopping potential of A relative to C is- a)3V
- b)_3V
- c)_1V
- d)4V
Correct answer is option 'B'. Can you explain this answer?
Photons with energy 5eV are incident on a cathode C, on a photoelectric cell. The maximum energy of the emitted photoelectrons is 2eV. When photons of energy 6eV are incident on C, no photoelectrons will reach the anode A if the stopping potential of A relative to C is
a)
3V
b)
_3V
c)
_1V
d)
4V
|
|
Geetika Shah answered |
When 5eV is incident the kinetic energy is 2eV it simply means the work function is W=5eV−2eV=3eV
Similarly, when 6eV is incident the kinetic energy should be 6eV−W=6eV−3eV=3eV
it simply means to stop them we need a negative potential at anode equal to 3eV/e=3V
So, the answer is −3V i.e. option B is correct.
Similarly, when 6eV is incident the kinetic energy should be 6eV−W=6eV−3eV=3eV
it simply means to stop them we need a negative potential at anode equal to 3eV/e=3V
So, the answer is −3V i.e. option B is correct.
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in- a)wavelength of radiation
- b)intensity of radiation
- c)frequency of radiation
- d)Both the wavelength and intensity of radiation
Correct answer is option 'C'. Can you explain this answer?
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in
a)
wavelength of radiation
b)
intensity of radiation
c)
frequency of radiation
d)
Both the wavelength and intensity of radiation
|
|
Arjun Singhania answered |
The kinetic energy of emitted photoelectrons should increase with the light amplitude. The rate of electron emission, which is proportional to the measured electric current, should increase as the light frequency is increased.
If h is Planck's constant is SI system, the momentum of a photon of wavelength 0.01 Å is- a)10-2 h
- b)h
- c)102 h
- d)1012 h
Correct answer is option 'D'. Can you explain this answer?
If h is Planck's constant is SI system, the momentum of a photon of wavelength 0.01 Å is
a)
10-2 h
b)
h
c)
102 h
d)
1012 h
|
|
Vivek Rana answered |
Momentum of photo, p=E/c=hν/c where E is the energy of a photon and c is the velocity of light.
∴ p= hc/cλ [∵ν=λc]
p=h/λ=h/(0.01×10−10)=1012h
∴ p= hc/cλ [∵ν=λc]
p=h/λ=h/(0.01×10−10)=1012h
In an atom, two electrons move around the nucleus in circular orbits of radii R and 4R. The ratio of the time taken by them to complete one revolution is : (neglect electric interaction)- a)1 : 4
- b)4 : 1
- c)1 : 8
- d) 8 : 1
Correct answer is option 'C'. Can you explain this answer?
In an atom, two electrons move around the nucleus in circular orbits of radii R and 4R. The ratio of the time taken by them to complete one revolution is : (neglect electric interaction)
a)
1 : 4
b)
4 : 1
c)
1 : 8
d)
8 : 1
|
|
Riya Banerjee answered |
Time period, T∝(R)3/2
∴ T1/T2 = (R1/R2 )3/2= (1/4)3/2=1/8
Thus, the ratio of time period is 1:8
∴ T1/T2 = (R1/R2 )3/2= (1/4)3/2=1/8
Thus, the ratio of time period is 1:8
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to- a)8 ×1014 HZ
- b)9 ×1014 HZ
- c)7 ×1014 HZ
- d)6 ×1014 HZ
Correct answer is option 'A'. Can you explain this answer?
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to
a)
8 ×1014 HZ
b)
9 ×1014 HZ
c)
7 ×1014 HZ
d)
6 ×1014 HZ
|
|
Anu Sharma answered |
The threshold frequency can be calculated using the formula:
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is- a)total momentum
- b)number of photons
- c)total energy
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is
a)
total momentum
b)
number of photons
c)
total energy
d)
None of the above
|
|
Rohan Singh answered |
Energy and momentum are conserved, resulting in a reduction of both for the scattered photon. ... This phenomenon could be handled as a collision between two particles—a photon and an electron at rest in the material. Energy and momentum are conserved in the collision.
Difference between nth and (n + 1)th Bohr's radius of `H' atom is equal to it's (n _ 1)th Bohr's radius. The value of n is- a)1
- b)2
- c)3
- d)4
Correct answer is option 'D'. Can you explain this answer?
Difference between nth and (n + 1)th Bohr's radius of `H' atom is equal to it's (n _ 1)th Bohr's radius. The value of n is
a)
1
b)
2
c)
3
d)
4
|
|
Rajesh Gupta answered |
We know that rn∝n2
Given: rn+1−rn=rn−1
∴(n+1)2−n2=(n−1)2
n=4
Given: rn+1−rn=rn−1
∴(n+1)2−n2=(n−1)2
n=4
An α – particle and a deutron are accelerated through the same potential difference. What will be the ratio of their de-Broglie wavelength?- a)1 / 3
- b)1 / 5
- c)1 / 4
- d)1 / 2
Correct answer is 'D'. Can you explain this answer?
An α – particle and a deutron are accelerated through the same potential difference. What will be the ratio of their de-Broglie wavelength?
a)
1 / 3
b)
1 / 5
c)
1 / 4
d)
1 / 2
|
|
Abhay Iyer answered |
Mass of alpha(a)= 4.
mass of deutron(d) =2.
wavelength=h/mv
Potential are same of both particle so their speed will be same .
Here, wavelength is inversely proportional to mass
so,
wavelength of alpha/wavelength of deutron = mass of deutron /mass of alpha.= 2/4=1/2
mass of deutron(d) =2.
wavelength=h/mv
Potential are same of both particle so their speed will be same .
Here, wavelength is inversely proportional to mass
so,
wavelength of alpha/wavelength of deutron = mass of deutron /mass of alpha.= 2/4=1/2
The de-Broglie wavelength of an electron is 1.0 nm. What is the retarding potential required to stop it?- a)1.5 V
- b)3.5 V
- c)6.5 V
- d)4.5 V
Correct answer is option 'A'. Can you explain this answer?
The de-Broglie wavelength of an electron is 1.0 nm. What is the retarding potential required to stop it?
a)
1.5 V
b)
3.5 V
c)
6.5 V
d)
4.5 V
|
|
Neha Sharma answered |
λ=h/P
Here P= √2mk
K=kinetic energy
λ=h/√2mk
By energy conversion
K=eVs
λ=h/√2meVs
√Vs=h/ λ√2me
Vs=h2/ λ2me
Vs=(6.626x10-34)2/(1x10-9)2x2x9.11x10-31xe
Where e=1.602x10-19
Vs=43.9x10-68/29.1x10-68
=1.508v
Vs=1.5v
Here P= √2mk
K=kinetic energy
λ=h/√2mk
By energy conversion
K=eVs
λ=h/√2meVs
√Vs=h/ λ√2me
Vs=h2/ λ2me
Vs=(6.626x10-34)2/(1x10-9)2x2x9.11x10-31xe
Where e=1.602x10-19
Vs=43.9x10-68/29.1x10-68
=1.508v
Vs=1.5v
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be- a)zero
- b)more than 2 volt
- c)2 Volt
- d)less than 2 volt
Correct answer is option 'B'. Can you explain this answer?
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be
a)
zero
b)
more than 2 volt
c)
2 Volt
d)
less than 2 volt
|
|
Geetika Shah answered |
The maximum kinetic energy for the photoelectrons is
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?- a)
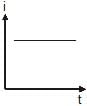
- b)
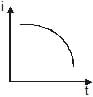
- c))

- d)
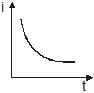
Correct answer is option 'D'. Can you explain this answer?
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?
a)
b)
c)
) 
d)
|
|
Geetika Shah answered |
Saturation current is the maximum current possible and it will be directly proportional to the number of number of electrons falling on collector plate per second which depend on number of photons
incident on the cathode as one photon contribute in one electron and the number of photons is actually
proportional to intensity which varies
As intensity I∝1/r2, where r is the distance
So the correct graph will be decreasing with power2 of distance and it will be rapidly decreasing with a higher value of r.
incident on the cathode as one photon contribute in one electron and the number of photons is actually
proportional to intensity which varies
As intensity I∝1/r2, where r is the distance
So the correct graph will be decreasing with power2 of distance and it will be rapidly decreasing with a higher value of r.
Cut off potentials for a metal in photoelectric effect for light of wavelength l1, l2 and l3 is found to be V1, V2 and V3 volts if V1, V2 and V3 are in Arithmetic Progression and l1, l2 and l3 will be- a)Arithmetic Progression
- b)Geometric Progression
- c)Harmonic Progression
- d)None
Correct answer is option 'C'. Can you explain this answer?
Cut off potentials for a metal in photoelectric effect for light of wavelength l1, l2 and l3 is found to be V1, V2 and V3 volts if V1, V2 and V3 are in Arithmetic Progression and l1, l2 and l3 will be
a)
Arithmetic Progression
b)
Geometric Progression
c)
Harmonic Progression
d)
None

|
Divey Sethi answered |
We know that,
eV=(hc/λ)-w
V=(hc/eλ)-(w/e)
Arithmetic progression =>V2=(V1+V2)/2
Now,
(hc/eλ2)-w/e=1/2[(hc/eλ1)-(w/e) +(hc/eλ3) -(w/e)]
=>1/ λ2=1/2[(1/ λ1)+(1/λ3)]
=>2/ λ2=1/ λ1 + 1/λ3
Hence the correct answer is harmonic Progression.
eV=(hc/λ)-w
V=(hc/eλ)-(w/e)
Arithmetic progression =>V2=(V1+V2)/2
Now,
(hc/eλ2)-w/e=1/2[(hc/eλ1)-(w/e) +(hc/eλ3) -(w/e)]
=>1/ λ2=1/2[(1/ λ1)+(1/λ3)]
=>2/ λ2=1/ λ1 + 1/λ3
Hence the correct answer is harmonic Progression.
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is- a)1.64 eV
- b)1.56 eV
- c)1.52 eV
- d)1.41 eV
Correct answer is option 'A'. Can you explain this answer?
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is
a)
1.64 eV
b)
1.56 eV
c)
1.52 eV
d)
1.41 eV
|
|
Neha Sharma answered |
The Stopping potential =2.5V.
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
In which case is electron emission from a metal not known?- a)Illuminating a metal surface with suitable light
- b)Applying a very strong magnetic field to a metal
- c)Applying a very strong electric field to a metal
- d)Heating the metal sufficiently
Correct answer is option 'B'. Can you explain this answer?
In which case is electron emission from a metal not known?
a)
Illuminating a metal surface with suitable light
b)
Applying a very strong magnetic field to a metal
c)
Applying a very strong electric field to a metal
d)
Heating the metal sufficiently

|
Divey Sethi answered |
The correct answer would be option B. Applying a very strong magnetic field.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is- a)1.6 ×1013Hz
- b)1.5 ×1013Hz
- c)1.7 ×1013Hz
- d)1.8 ×1013Hz
Correct answer is option 'B'. Can you explain this answer?
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is
a)
1.6 ×1013Hz
b)
1.5 ×1013Hz
c)
1.7 ×1013Hz
d)
1.8 ×1013Hz
|
|
Lavanya Menon answered |
Frequency=C/ λ
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
A image of the sun of formed by a lens of focal-length of 30 cm on the metal surface of a photo-electric cell and a photo-electrci current is produced. The lens forming the image is then replaced by another of the same diameter but of focal length 15 cm. The photo-electric current in this case is- a)I/2
- b)I
- c)2I
- d)4I
Correct answer is option 'B'. Can you explain this answer?
A image of the sun of formed by a lens of focal-length of 30 cm on the metal surface of a photo-electric cell and a photo-electrci current is produced. The lens forming the image is then replaced by another of the same diameter but of focal length 15 cm. The photo-electric current in this case is
a)
I/2
b)
I
c)
2I
d)
4I

|
Knowledge Hub answered |
Lenses of the same diameter collect equal amounts of light.
Intensity is the measure of amount of light collected, hence the intensity remains the same.
Intensity is measured by the photoelectric current. So the photoelectric current would also remain the same.
Intensity is the measure of amount of light collected, hence the intensity remains the same.
Intensity is measured by the photoelectric current. So the photoelectric current would also remain the same.
Of the following moving with the same momentum, the one which has the largest wavelength is- a)a proton
- b)α -particle
- c)an electron
- d)all have the same de Broglie wavelength
Correct answer is option 'D'. Can you explain this answer?
Of the following moving with the same momentum, the one which has the largest wavelength is
a)
a proton
b)
α -particle
c)
an electron
d)
all have the same de Broglie wavelength
|
|
Gaurav Kumar answered |
De Broglie wavelength = h/mv,
where h = plancks constant, mv = momentum.
As they move with same momentum, de Broglie wavelength remains constant for all.
where h = plancks constant, mv = momentum.
As they move with same momentum, de Broglie wavelength remains constant for all.
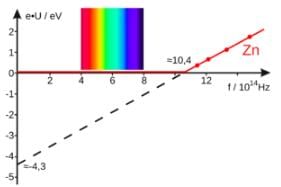
The stopping potential for the photo electrons emitted from a metal surface of work function 1.7eV is 10.4 V. Identify the energy levels corresponding to the transitions in hydrogen atom which will result in emission of wavelength equal to that of incident radiation for the above photoelectric effect- a)n = 3 to 1
- b)n = 3 to 2
- c)n = 2 to 1
- d)n = 4 to 1
Correct answer is option 'A'. Can you explain this answer?
The stopping potential for the photo electrons emitted from a metal surface of work function 1.7eV is 10.4 V. Identify the energy levels corresponding to the transitions in hydrogen atom which will result in emission of wavelength equal to that of incident radiation for the above photoelectric effect
a)
n = 3 to 1
b)
n = 3 to 2
c)
n = 2 to 1
d)
n = 4 to 1
|
|
Gaurav Kumar answered |
As we know that the stopping potential of the photoelectron is equal to the maximum kinetic energy of the photoelectron,
KEmax=10.4V
Now, in photoelectric effect,
Energy of incident radiation (Ein) = work function + K.Emax
⇒ Ein=1.7+10.4
⇒ Ein=12.1eV
Now, for 0 hydrogen atom,
Energy of first energy level, E1=−13.6eV
Energy of second energy level, E2=−3.4eV
Energy of third energy level, E3=−1.5eV
Hence, a transition from third to first energy level will result in emission of radiation of energy = E3−E1=12.1eV which is same as the energy of incident radiation of above photoelectric effect.
Thus, correct answer is n=3 to 1
KEmax=10.4V
Now, in photoelectric effect,
Energy of incident radiation (Ein) = work function + K.Emax
⇒ Ein=1.7+10.4
⇒ Ein=12.1eV
Now, for 0 hydrogen atom,
Energy of first energy level, E1=−13.6eV
Energy of second energy level, E2=−3.4eV
Energy of third energy level, E3=−1.5eV
Hence, a transition from third to first energy level will result in emission of radiation of energy = E3−E1=12.1eV which is same as the energy of incident radiation of above photoelectric effect.
Thus, correct answer is n=3 to 1
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is- a)2 V
- b)6 V
- c)4 V
- d)10 V
Correct answer is option 'C'. Can you explain this answer?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is
a)
2 V
b)
6 V
c)
4 V
d)
10 V
|
|
Rajeev Saxena answered |
Stopping potential is nothing but the maximum kinetic energy of electros which get emitted during photoelectric effect.
So the stopping potential = maximum kinetic energy = 4eV
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4eV. The stopping potential is Volts is- a)2
- b)4
- c)6
- d)10
Correct answer is option 'B'. Can you explain this answer?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4eV. The stopping potential is Volts is
a)
2
b)
4
c)
6
d)
10
|
|
Naina Datta answered |
Explanation:
When a photon of energy hν falls on the surface of a metal, an electron is emitted if the energy of the photon is greater than or equal to the work function of the metal.
The maximum kinetic energy of the emitted photoelectrons is given by:
K.E. max = hν - φ
where h is Planck’s constant, ν is the frequency of the incident radiation, and φ is the work function of the metal.
Given that the energy of the incident photons is 6 eV and the maximum kinetic energy of the emitted photoelectrons is 4 eV, we have:
K.E. max = 4 eV
hν = 6 eV
Substituting these values in the above equation, we get:
4 eV = 6 eV - φ
φ = 2 eV
Therefore, the work function of the metal is 2 eV.
When a potential difference (stopping potential) V is applied between the metal surface and the collector electrode, the photoelectrons are brought to rest and the maximum kinetic energy of the emitted photoelectrons is equal to the potential energy gained by the electrons in moving from the metal surface to the collector electrode.
Therefore, we have:
K.E. max = eV
where e is the electronic charge.
Substituting the value of K.E. max and e in the above equation, we get:
4 eV = eV
V = 4 V
Hence, the stopping potential is 4 volts. Therefore, option B is the correct answer.
When a photon of energy hν falls on the surface of a metal, an electron is emitted if the energy of the photon is greater than or equal to the work function of the metal.
The maximum kinetic energy of the emitted photoelectrons is given by:
K.E. max = hν - φ
where h is Planck’s constant, ν is the frequency of the incident radiation, and φ is the work function of the metal.
Given that the energy of the incident photons is 6 eV and the maximum kinetic energy of the emitted photoelectrons is 4 eV, we have:
K.E. max = 4 eV
hν = 6 eV
Substituting these values in the above equation, we get:
4 eV = 6 eV - φ
φ = 2 eV
Therefore, the work function of the metal is 2 eV.
When a potential difference (stopping potential) V is applied between the metal surface and the collector electrode, the photoelectrons are brought to rest and the maximum kinetic energy of the emitted photoelectrons is equal to the potential energy gained by the electrons in moving from the metal surface to the collector electrode.
Therefore, we have:
K.E. max = eV
where e is the electronic charge.
Substituting the value of K.E. max and e in the above equation, we get:
4 eV = eV
V = 4 V
Hence, the stopping potential is 4 volts. Therefore, option B is the correct answer.
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will- a)be doubled
- b)halved
- c)become more than doubled
- d)become less than double
Correct answer is option 'C'. Can you explain this answer?
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will
a)
be doubled
b)
halved
c)
become more than doubled
d)
become less than double
|
|
Om Desai answered |
The maximum kinetic energy for the photoelectrons is
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
eV0=hν−ϕ ...................(1)
where, V0 is the stopping potential and e is the electronic charge.
When, the frequency of light in a photoelectric experiment is doubled,
eV0′=2hν−ϕ
eV0′=2[hν−(ϕ/2)].........................(2)
From the above two equations we can say that the K.E. in (2) is more than double of K.E in (1). Hence, when the frequency of light in a photoelectric experiment is doubled, the stopping potential becomes more than double.
So, the answer is option (C).
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
eV0=hν−ϕ ...................(1)
where, V0 is the stopping potential and e is the electronic charge.
When, the frequency of light in a photoelectric experiment is doubled,
eV0′=2hν−ϕ
eV0′=2[hν−(ϕ/2)].........................(2)
From the above two equations we can say that the K.E. in (2) is more than double of K.E in (1). Hence, when the frequency of light in a photoelectric experiment is doubled, the stopping potential becomes more than double.
So, the answer is option (C).
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation- a)is independent of the radiation intensity
- b)is inversely proportional to radiation intensity
- c)is proportional to radiation intensity
- d)none
Correct answer is option 'A'. Can you explain this answer?
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation
a)
is independent of the radiation intensity
b)
is inversely proportional to radiation intensity
c)
is proportional to radiation intensity
d)
none
|
|
Shreya Singh answered |
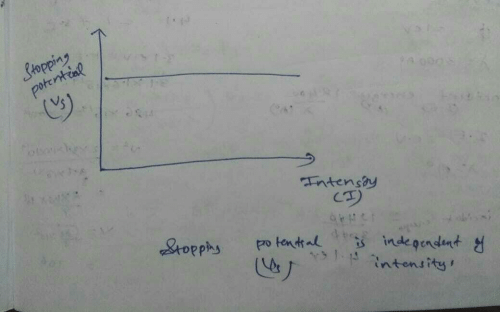
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is- a)7 ×10-17 J
- b)8 ×10−17 J
- c)6 ×10−17 J
- d)9 ×10−17 J
Correct answer is option 'B'. Can you explain this answer?
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is
a)
7 ×10-17 J
b)
8 ×10−17 J
c)
6 ×10−17 J
d)
9 ×10−17 J
|
|
Om Desai answered |
Voltage across the electrodes of a cathode ray gun, V=500V
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
Photons can be- a)scattered
- b)deflected by magnetic fields
- c)deflected by electric fields
- d)none
Correct answer is option 'A'. Can you explain this answer?
Photons can be
a)
scattered
b)
deflected by magnetic fields
c)
deflected by electric fields
d)
none
|
|
Rajeev Saxena answered |
As this electron changes orbit, its energy is reduced, and the excess energy is given off in the form of a photon, called a “characteristic photon.” In pair production, photon energies greater than 1.02 MeV interact with the strong electric field of the nucleus and lose all incident energy.
By increasing the intensity of incident light keeping frequency (v > v0) fixed on the surface of metal- a)kinetic energy of the photoelectrons increases
- b)number of emitted electrons increases
- c)kinetic energy and number of electrons increases
- d)no effect
Correct answer is option 'B'. Can you explain this answer?
By increasing the intensity of incident light keeping frequency (v > v0) fixed on the surface of metal
a)
kinetic energy of the photoelectrons increases
b)
number of emitted electrons increases
c)
kinetic energy and number of electrons increases
d)
no effect
|
|
Geetika Shah answered |
The number of photoelectrons emitted per second from a photosensitive plate is directly proportional to the intensity of the incident radiation. ... For the same frequency of light and increased intensity, the saturation current is found to increase, but the cut-off potential is found to remain constant.
The number of electrons also changes because of the probability that each photon results in an emitted electron are a function of photon energy. If the intensity of the incident radiation of a given frequency is increased, there is no effect on the kinetic energy of each photo electron.
The number of electrons also changes because of the probability that each photon results in an emitted electron are a function of photon energy. If the intensity of the incident radiation of a given frequency is increased, there is no effect on the kinetic energy of each photo electron.
The radius of Bohr's first orbit is a0. The electron in nth orbit has a radius- a)na0
- b)a0/n
- c)n2a0
- d)a0/n2
Correct answer is option 'C'. Can you explain this answer?
The radius of Bohr's first orbit is a0. The electron in nth orbit has a radius
a)
na0
b)
a0/n
c)
n2a0
d)
a0/n2
|
|
Gaurav Kumar answered |
Radius of nth orbital
rn= ϵ0n2h2/ πmZe2
Wherein
=rn∝ n2 /Z
= ϵ0h2/πme2 =0.529
r=(n2/Z)a0
For Z=1 r=n2a0
rn= ϵ0n2h2/ πmZe2
Wherein
=rn∝ n2 /Z
= ϵ0h2/πme2 =0.529
r=(n2/Z)a0
For Z=1 r=n2a0
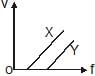
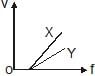
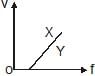
In a photoelectric experiment, electrons are ejected from metals X and Y by light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X ; which one of the following graphs best illustrates the expected results ?- a)
- b)
- c)
- d)

Correct answer is option 'A'. Can you explain this answer?
In a photoelectric experiment, electrons are ejected from metals X and Y by light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X ; which one of the following graphs best illustrates the expected results ?
a)
b)
c)
d)
|
|
Nikita Singh answered |
The answer is option A.
First, the gradient of the graph cannot change (always = h/e ), so answers are A or D.
If Y has a greater work function than X, then the graph for Y should have a more negative y-intercept. therefore figure in option A depicts the concept.
First, the gradient of the graph cannot change (always = h/e ), so answers are A or D.
If Y has a greater work function than X, then the graph for Y should have a more negative y-intercept. therefore figure in option A depicts the concept.
In Photoelectric effect- a)electrical energy is converted magnetic field energy
- b)electrical energy is converted into light energy
- c)light is converted into electrical energy
- d)electrical energy is converted into heat
Correct answer is option 'C'. Can you explain this answer?
In Photoelectric effect
a)
electrical energy is converted magnetic field energy
b)
electrical energy is converted into light energy
c)
light is converted into electrical energy
d)
electrical energy is converted into heat
|
|
Nikita Singh answered |
Photoelectric cell or photocell, device whose electrical characteristics (e.g., current, voltage, or resistance) vary when light is incident upon it. The most common type consists of two electrodes separated by a light-sensitive semiconductor material.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
A photon is- a)a positive charged particle
- b)a quantum of light energy
- c)an instrument for measuring light intensity
- d)a quantum of matter
Correct answer is option 'B'. Can you explain this answer?
A photon is
a)
a positive charged particle
b)
a quantum of light energy
c)
an instrument for measuring light intensity
d)
a quantum of matter

|
Knowledge Hub answered |
A particle representing a quantum of light or other electromagnetic radiation. A photon carries energy proportional to the radiation frequency but has zero rest mass.
The angular momentum of an electron in the hydrogen atom is 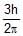 . Here h is Planck's constant. The kinetic energy of this electron is
. Here h is Planck's constant. The kinetic energy of this electron is- a)4.53 eV
- b)1.51 eV
- c)3.4 eV
- d)6.8 eV
Correct answer is option 'B'. Can you explain this answer?
The angular momentum of an electron in the hydrogen atom is 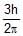 . Here h is Planck's constant. The kinetic energy of this electron is
. Here h is Planck's constant. The kinetic energy of this electron is
a)
4.53 eV
b)
1.51 eV
c)
3.4 eV
d)
6.8 eV

|
Ayan Mondal answered |
We know that the formula of the angular momentum of an electron in n'th orbit is L=nh/2π. Here n: principal quantum no. Thus as per the question n=3. Hance the kinetic energy of the electron in third orbit is 1.51eV.
in photoelectric effect, the photoelectric current- a)both on intensity and frequency of incident beam
- b)increases when frequency of incident photons increases
- c)decreases when frequency of incident photons increases
- d)does not depend on photon frequency but only on intensity of incident
Correct answer is option 'D'. Can you explain this answer?
in photoelectric effect, the photoelectric current
a)
both on intensity and frequency of incident beam
b)
increases when frequency of incident photons increases
c)
decreases when frequency of incident photons increases
d)
does not depend on photon frequency but only on intensity of incident
|
|
Arun Khanna answered |
In photoelectric effect,when light incident on metal surface the no. Of electron emitted is depends on intensity of light and speed of Electron is depends on frequency of incidents light. Photoelectric current is due to the ejection of photo electrons.
Photoelectric effect is- a)emission of electrons by glass when illuminated by light of suitable frequency
- b)emission of electrons by metals when heated
- c)emission of electrons by insulators when illuminated by light of suitable frequency
- d)emission of electrons by metals when illuminated by light of suitable frequency
Correct answer is option 'D'. Can you explain this answer?
Photoelectric effect is
a)
emission of electrons by glass when illuminated by light of suitable frequency
b)
emission of electrons by metals when heated
c)
emission of electrons by insulators when illuminated by light of suitable frequency
d)
emission of electrons by metals when illuminated by light of suitable frequency

|
Prasenjit Malik answered |
Understanding the Photoelectric Effect
The photoelectric effect is a fundamental phenomenon in physics, describing how light interacts with matter, particularly metals. Here’s a detailed explanation of why option 'D' is the correct choice.
Definition of the Photoelectric Effect
- The photoelectric effect refers to the emission of electrons from a metal surface when it is exposed to light of suitable frequency (or energy).
Key Characteristics
- Electron Emission: When light shines on a metal, photons (light particles) can impart energy to the electrons in the metal.
- Threshold Frequency: Each metal has a specific threshold frequency. If the frequency of the incoming light is higher than this threshold, electrons are emitted. If it is lower, no emission occurs regardless of light intensity.
Why Option 'D' is Correct
- Metals and Electron Emission: Metals have free electrons that can be easily displaced. When illuminated by light of suitable frequency, these electrons can absorb sufficient energy to overcome the binding forces holding them in the metal, leading to their emission.
- Other Options Explained:
- Option A: Glass does not emit electrons; it is an insulator.
- Option B: Heating metals causes thermal emission, not the photoelectric effect.
- Option C: Insulators do not typically emit electrons upon illumination because their electrons are tightly bound.
Conclusion
- The photoelectric effect is a crucial concept in understanding quantum mechanics and the dual nature of light. It highlights how light can behave as both a wave and a particle, contributing to advancements in technology, such as photoelectric cells and solar panels. Thus, option 'D' accurately describes this phenomenon.
The photoelectric effect is a fundamental phenomenon in physics, describing how light interacts with matter, particularly metals. Here’s a detailed explanation of why option 'D' is the correct choice.
Definition of the Photoelectric Effect
- The photoelectric effect refers to the emission of electrons from a metal surface when it is exposed to light of suitable frequency (or energy).
Key Characteristics
- Electron Emission: When light shines on a metal, photons (light particles) can impart energy to the electrons in the metal.
- Threshold Frequency: Each metal has a specific threshold frequency. If the frequency of the incoming light is higher than this threshold, electrons are emitted. If it is lower, no emission occurs regardless of light intensity.
Why Option 'D' is Correct
- Metals and Electron Emission: Metals have free electrons that can be easily displaced. When illuminated by light of suitable frequency, these electrons can absorb sufficient energy to overcome the binding forces holding them in the metal, leading to their emission.
- Other Options Explained:
- Option A: Glass does not emit electrons; it is an insulator.
- Option B: Heating metals causes thermal emission, not the photoelectric effect.
- Option C: Insulators do not typically emit electrons upon illumination because their electrons are tightly bound.
Conclusion
- The photoelectric effect is a crucial concept in understanding quantum mechanics and the dual nature of light. It highlights how light can behave as both a wave and a particle, contributing to advancements in technology, such as photoelectric cells and solar panels. Thus, option 'D' accurately describes this phenomenon.
Let nr and nb be respectively the number of photons emitted by a red bulb and a blue bulb of equal power in a given time.- a)nr = nb
- b)nr < nb
- c)nr > nb
- d)data insufficient
Correct answer is option 'C'. Can you explain this answer?
Let nr and nb be respectively the number of photons emitted by a red bulb and a blue bulb of equal power in a given time.
a)
nr = nb
b)
nr < nb
c)
nr > nb
d)
data insufficient
|
|
Gaurav Kumar answered |
Since, Pr=Pb
r for red and b for blue.
Pr=Pb
or, nr× (hc/λr) =nb× (hc/λb)
or, (nr/nb)= λr/λb
Since, the wavelength of red bulb is greater than the wavelength of blue bulb.
or, nr>nb
r for red and b for blue.
Pr=Pb
or, nr× (hc/λr) =nb× (hc/λb)
or, (nr/nb)= λr/λb
Since, the wavelength of red bulb is greater than the wavelength of blue bulb.
or, nr>nb
If the work function of a material is 2eV, then minimum frequency of light required to emit photo-electrons is - a)5.0 ×1015Hz
- b)4.6 ×1015Hz
- c)4.6 ×1014Hz
- d)5.0 ×1014Hz
Correct answer is option 'C'. Can you explain this answer?
If the work function of a material is 2eV, then minimum frequency of light required to emit photo-electrons is
a)
5.0 ×1015Hz
b)
4.6 ×1015Hz
c)
4.6 ×1014Hz
d)
5.0 ×1014Hz
|
|
Lavanya Menon answered |
Φ= hνλ => 2eV= 6.626 x 10-34 x ν
2 x 1.6 x 10-19= 6.626 x 10-34 x ν
On solving we get ν = 4.6 x 1014 Hz.
Consider the spectral line resulting from the transition n = 2 → n = 1 in the atoms and ions given below. The shortest wavelength is produced by :- a)Hydrogen atom
- b)Deuterium atom
- c)Singly ionized helium
- d)Doubly ionized lithium
Correct answer is option 'D'. Can you explain this answer?
Consider the spectral line resulting from the transition n = 2 → n = 1 in the atoms and ions given below. The shortest wavelength is produced by :
a)
Hydrogen atom
b)
Deuterium atom
c)
Singly ionized helium
d)
Doubly ionized lithium
|
|
Vasanth Kumar answered |
When a photon of light collides with a metal surface, number of electrons, (if any) coming out is- a)only one
- b)only two
- c)infinite
- d)depends upon factors
Correct answer is option 'A'. Can you explain this answer?
When a photon of light collides with a metal surface, number of electrons, (if any) coming out is
a)
only one
b)
only two
c)
infinite
d)
depends upon factors
|
|
Om Desai answered |
When photon strikes with the electron it completely transfers it’s energy to the electron as during photoelectric experiment the threshold frequency required is used by the electron to eject from the atom which is also called the work function and the remaining energy in electron is kinetic energy which can be measured. Now (before collision) since photon is a particle it must have mass and thus it has energy equivalent to E=mc2 .
After collision when it completely transfers it’s energy to electron thus E=0
And therefore 0=mc2 thus i guess photon vanishes.
After collision when it completely transfers it’s energy to electron thus E=0
And therefore 0=mc2 thus i guess photon vanishes.
In the above experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then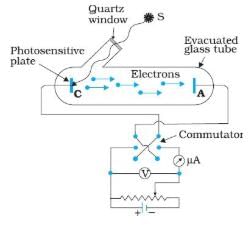
- a)photocurrent decreases nonlinearly with intensity
- b)photocurrent decreases linearly with intensity
- c)photocurrent remains same with intensity
- d)photocurrent increases linearly with intensity
Correct answer is option 'D'. Can you explain this answer?
In the above experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then

a)
photocurrent decreases nonlinearly with intensity
b)
photocurrent decreases linearly with intensity
c)
photocurrent remains same with intensity
d)
photocurrent increases linearly with intensity

|
Knowledge Hub answered |
The number of electrons emitted per second is observed to be directly proportional to the intensity of light. “Ok, so light is a wave and has energy. It takes electrons out of a metal, what is so special about that!” First of all, when the intensity of light is increased, we should see an increase in the photocurrent (number of photoelectrons emitted). Right?
As we see, this only happens above a specific value of frequency, known as the threshold frequency. Below this threshold frequency, the intensity of light has no effect on the photocurrent! In fact, there is no photocurrent at all, however high the intensity of light is.
The graph between the photoelectric current and the intensity of light is a straight line when the frequency of light used is above a specific minimum threshold value.
As we see, this only happens above a specific value of frequency, known as the threshold frequency. Below this threshold frequency, the intensity of light has no effect on the photocurrent! In fact, there is no photocurrent at all, however high the intensity of light is.

The graph between the photoelectric current and the intensity of light is a straight line when the frequency of light used is above a specific minimum threshold value.
Energy of a photon of green light of wavelength 5500 is (given: h = 6.62 ×10−34Js−1) approximately- a)3.01eV
- b)2.81ev
- c)2.26 eV
- d)2.93 eV
Correct answer is 'C'. Can you explain this answer?
Energy of a photon of green light of wavelength 5500 is (given: h = 6.62 ×10−34Js−1) approximately
a)
3.01eV
b)
2.81ev
c)
2.26 eV
d)
2.93 eV
|
|
Rajesh Gupta answered |
As we know,
the formula for energy of photon in terms of wavelength is,, E = hc/ λ
where, E = energy of photon
h = Planck's constant =
= 6.63×10-34 Js
c = speed of light
= 3×108 m/s
and lambda = wavelength
so,
E = [6.63×10-34 J. s×3×108 m/s]/5500×10-10m
= [0.36×10-26]/10-8
= 0.36×10-18
= 3.6×10-19 J
Or, 2.26ev
the formula for energy of photon in terms of wavelength is,, E = hc/ λ
where, E = energy of photon
h = Planck's constant =
= 6.63×10-34 Js
c = speed of light
= 3×108 m/s
and lambda = wavelength
so,
E = [6.63×10-34 J. s×3×108 m/s]/5500×10-10m
= [0.36×10-26]/10-8
= 0.36×10-18
= 3.6×10-19 J
Or, 2.26ev
The work function of metal is 1 eV. Light of wavelength 3000 Å is incident on this metal surface. The velocity of emitted photo-electrons will be- a) 10 m/sec
- b)1 × 103 m / sec
- c)1 × 104 m / sec
- d)1× 106 m / sec
Correct answer is option 'D'. Can you explain this answer?
The work function of metal is 1 eV. Light of wavelength 3000 Å is incident on this metal surface. The velocity of emitted photo-electrons will be
a)
10 m/sec
b)
1 × 103 m / sec
c)
1 × 104 m / sec
d)
1× 106 m / sec
|
|
Sai Mehta answered |
Understanding the Problem
The work function of the metal is given as 1 eV, and light of wavelength 3000 Å (Angstroms) is incident on it. We need to find the velocity of the emitted photoelectrons.
Key Concepts
- Work Function (Φ): The minimum energy required to remove an electron from the surface of a metal, given in electron volts (eV).
- Energy of Photons (E): The energy of the incident light can be calculated using the formula:
E = hc/λ
where:
- h = Planck's constant (4.135667696 × 10^-15 eV·s)
- c = Speed of light (3 × 10^8 m/s)
- λ = Wavelength (in meters)
Calculating Energy of Incident Light
1. Convert Wavelength:
3000 Å = 3000 × 10^-10 m = 3 × 10^-7 m
2. Calculate Energy (E):
E = (4.135667696 × 10^-15 eV·s) × (3 × 10^8 m/s) / (3 × 10^-7 m)
E = 4.135667696 eV
Photoelectric Effect
- Kinetic Energy (KE): The kinetic energy of the emitted electrons is given by:
KE = E - Φ
KE = 4.135667696 eV - 1 eV = 3.135667696 eV
Calculating Velocity of Electrons
- Using Kinetic Energy Formula:
KE = (1/2)mv², where m is the mass of an electron (approximately 9.11 × 10^-31 kg).
- Rearranging gives:
v = sqrt(2 * KE / m)
- Converting KE to Joules (1 eV = 1.6 × 10^-19 J):
KE = 3.135667696 eV × 1.6 × 10^-19 J/eV = 5.016 e-19 J
- Now, substituting the values:
v = sqrt(2 * 5.016 e-19 J / 9.11 e-31 kg)
v ≈ 1 × 10^6 m/s
Conclusion
Thus, the velocity of the emitted photoelectrons is approximately 1 × 10^6 m/s, confirming that the correct answer is option 'D'.
The work function of the metal is given as 1 eV, and light of wavelength 3000 Å (Angstroms) is incident on it. We need to find the velocity of the emitted photoelectrons.
Key Concepts
- Work Function (Φ): The minimum energy required to remove an electron from the surface of a metal, given in electron volts (eV).
- Energy of Photons (E): The energy of the incident light can be calculated using the formula:
E = hc/λ
where:
- h = Planck's constant (4.135667696 × 10^-15 eV·s)
- c = Speed of light (3 × 10^8 m/s)
- λ = Wavelength (in meters)
Calculating Energy of Incident Light
1. Convert Wavelength:
3000 Å = 3000 × 10^-10 m = 3 × 10^-7 m
2. Calculate Energy (E):
E = (4.135667696 × 10^-15 eV·s) × (3 × 10^8 m/s) / (3 × 10^-7 m)
E = 4.135667696 eV
Photoelectric Effect
- Kinetic Energy (KE): The kinetic energy of the emitted electrons is given by:
KE = E - Φ
KE = 4.135667696 eV - 1 eV = 3.135667696 eV
Calculating Velocity of Electrons
- Using Kinetic Energy Formula:
KE = (1/2)mv², where m is the mass of an electron (approximately 9.11 × 10^-31 kg).
- Rearranging gives:
v = sqrt(2 * KE / m)
- Converting KE to Joules (1 eV = 1.6 × 10^-19 J):
KE = 3.135667696 eV × 1.6 × 10^-19 J/eV = 5.016 e-19 J
- Now, substituting the values:
v = sqrt(2 * 5.016 e-19 J / 9.11 e-31 kg)
v ≈ 1 × 10^6 m/s
Conclusion
Thus, the velocity of the emitted photoelectrons is approximately 1 × 10^6 m/s, confirming that the correct answer is option 'D'.
Radiation of two photon energies twice and five times the work function of metal are incident sucessively on the metal surface. The ratio of the maximum velocity of photoelectrons emitted is the two cases will be- a)1 : 2
- b)2 : 1
- c)1 : 4
- d)4 : 1
Correct answer is option 'A'. Can you explain this answer?
Radiation of two photon energies twice and five times the work function of metal are incident sucessively on the metal surface. The ratio of the maximum velocity of photoelectrons emitted is the two cases will be
a)
1 : 2
b)
2 : 1
c)
1 : 4
d)
4 : 1
|
|
Ram Mohith answered |
Let the maximum velocities of the photoelectrons in each case be v and u respectively and the work function of the metal be w
• Energy of photon = 2w
2w - w = mv²/2 => mv²/2 = w
• Energy of the photon = 5w
5w - w = mu²/2 => mu²/2 = 4w
On dividing,
(v/u)² = 1/4
=> v/u = 1/2
• Energy of photon = 2w
2w - w = mv²/2 => mv²/2 = w
• Energy of the photon = 5w
5w - w = mu²/2 => mu²/2 = 4w
On dividing,
(v/u)² = 1/4
=> v/u = 1/2
Chapter doubts & questions for Matter Waves: De Broglie Wavelength - Physics for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Matter Waves: De Broglie Wavelength - Physics for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Physics for EmSAT Achieve
208 videos|329 docs|212 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup