All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Emission Spectra for EmSAT Achieve Exam
Among the species given, the one possessing charge-transfer transition in the visible region is:
- a)Th(C2O4)3
- b)HgI2
- c)ZnS
- d)[Cr(NCS)6]3-
Correct answer is option 'D'. Can you explain this answer?
Among the species given, the one possessing charge-transfer transition in the visible region is:
a)
Th(C2O4)3
b)
HgI2
c)
ZnS
d)
[Cr(NCS)6]3-

|
Sparsh Menon answered |
Charge-transfer transition in visible regionIntroduction:Charge-transfer transition is a type of electronic transition that occurs between two different molecular entities or between different parts of the same molecule. In this transition, an electron is transferred from one molecular entity to another, resulting in a change in the oxidation state of the entities involved. This type of transition is commonly observed in transition metal complexes and can occur in the visible region of the electromagnetic spectrum.Species possessing charge-transfer transition in the visible region:Among the species given, the one possessing charge-transfer transition in the visible region is [Cr(NCS)6]3-. This is because of the following reasons:1. Chromium in [Cr(NCS)6]3-:Chromium in [Cr(NCS)6]3- is in the +3 oxidation state, which makes it a transition metal complex. Transition metal complexes are known to exhibit charge-transfer transitions, and these transitions often occur in the visible region of the electromagnetic spectrum.2. NCS- ligands:The NCS- ligands in [Cr(NCS)6]3- are known to be good pi-acceptors. This means that they can accept electrons from the chromium ion, resulting in a charge-transfer transition. This transition is often observed in the visible region of the electromagnetic spectrum, making [Cr(NCS)6]3- a good candidate for possessing charge-transfer transition in the visible region.3. Visible region:The visible region of the electromagnetic spectrum is defined as the range of wavelengths between 400 and 700 nm. Charge-transfer transitions involving transition metal complexes often occur in this region, making it likely that [Cr(NCS)6]3- exhibits a charge-transfer transition in the visible region.Conclusion:In conclusion, the species that possesses charge-transfer transition in the visible region is [Cr(NCS)6]3-. This is due to the presence of chromium in the +3 oxidation state and the NCS- ligands, which are known to be good pi-acceptors. Charge-transfer transitions involving transition metal complexes often occur in the visible region of the electromagnetic spectrum, making [Cr(NCS)6]3- a good candidate for possessing charge-transfer transition in the visible region.
The lowest energy d-d transition in the Cr(III) complexes varies in the order- a)CrCl63- < Cr(H2O)63+ < Cr(en)33+ < Cr(CN)63-
- b)CrCl63- < Cr(en)33+ < Cr(H2O)63+ < Cr(CN)63-
- c)Cr(CN)63- < CrCl63- < Cr(H2O)63+ < Cr(en)33+
- d)Cr(H2O)63+ < Cr(en)33+ < CrCl63- < Cr(CN)63-
Correct answer is option 'A'. Can you explain this answer?
The lowest energy d-d transition in the Cr(III) complexes varies in the order
a)
CrCl63- < Cr(H2O)63+ < Cr(en)33+ < Cr(CN)63-
b)
CrCl63- < Cr(en)33+ < Cr(H2O)63+ < Cr(CN)63-
c)
Cr(CN)63- < CrCl63- < Cr(H2O)63+ < Cr(en)33+
d)
Cr(H2O)63+ < Cr(en)33+ < CrCl63- < Cr(CN)63-

|
Anushka Basak answered |
B) Cr(H2O)63+ c) CrF63- d) Cr(NH3)63+
The red color of oxyhaemoglobin is mainly due to the- a)d-d transition
- b)metal to ligand charge transfer transition
- c)ligand to metal charge transfer transition
- d)Intra Ligand π-π* transition
Correct answer is option 'D'. Can you explain this answer?
The red color of oxyhaemoglobin is mainly due to the
a)
d-d transition
b)
metal to ligand charge transfer transition
c)
ligand to metal charge transfer transition
d)
Intra Ligand π-π* transition

|
Dipanjan Sharma answered |
None of the above options are correct. The red color of oxyhaemoglobin is due to the absorption of light in the visible spectrum by the heme group, which consists of a porphyrin ring complexed with an iron ion. Specifically, the red color is due to the Soret band, which arises from a d-d transition within the iron ion.
Which of the following compounds shown intervalence charge transfer transition- a)Pb3O4
- b)KCr2O7
- c)KFe[Fe(CN)6]
- d)Mn2(CO)10
Correct answer is option 'A,C'. Can you explain this answer?
Which of the following compounds shown intervalence charge transfer transition
a)
Pb3O4
b)
KCr2O7
c)
KFe[Fe(CN)6]
d)
Mn2(CO)10

|
Preethi Joshi answered |
**Intervalence Charge Transfer Transitions**
Intervalence charge transfer (IVCT) transitions occur when there is a transfer of electrons between two metal centers in a compound. These transitions typically involve metal ions with different oxidation states, resulting in a difference in the formal charges of the metal centers.
**a) Pb3O4**
In Pb3O4, lead (Pb) can exist in two different oxidation states: +2 and +4. The compound contains both Pb(II) and Pb(IV) ions, which allows for the possibility of IVCT transitions. The IVCT transitions in Pb3O4 involve the transfer of electrons between the Pb(II) and Pb(IV) ions. These transitions can result in the absorption of light in the visible range, leading to the compound's characteristic brown color.
**c) KFe[Fe(CN)6]**
KFe[Fe(CN)6] is a coordination compound that contains both Fe(II) and Fe(III) ions. The Fe(II) ion has a +2 oxidation state, while the Fe(III) ion has a +3 oxidation state. The IVCT transitions in KFe[Fe(CN)6] involve the transfer of electrons between the Fe(II) and Fe(III) ions. These transitions can result in the absorption of light in the visible range, leading to the compound's characteristic yellow color.
**b) KCr2O7**
KCr2O7 is a compound that contains chromium (Cr) in the +6 oxidation state. While it does involve a difference in oxidation states, it does not contain two metal ions that can undergo IVCT transitions. Therefore, KCr2O7 does not exhibit IVCT transitions.
**d) Mn2(CO)10**
Mn2(CO)10 is a coordination compound that contains manganese (Mn) in the +2 oxidation state. It does not contain two metal ions with different oxidation states that can undergo IVCT transitions. Therefore, Mn2(CO)10 does not exhibit IVCT transitions.
In conclusion, the compounds that exhibit IVCT transitions are Pb3O4 (option A) and KFe[Fe(CN)6] (option C). These compounds contain metal ions with different oxidation states, allowing for the transfer of electrons and absorption of light in the visible range.
Intervalence charge transfer (IVCT) transitions occur when there is a transfer of electrons between two metal centers in a compound. These transitions typically involve metal ions with different oxidation states, resulting in a difference in the formal charges of the metal centers.
**a) Pb3O4**
In Pb3O4, lead (Pb) can exist in two different oxidation states: +2 and +4. The compound contains both Pb(II) and Pb(IV) ions, which allows for the possibility of IVCT transitions. The IVCT transitions in Pb3O4 involve the transfer of electrons between the Pb(II) and Pb(IV) ions. These transitions can result in the absorption of light in the visible range, leading to the compound's characteristic brown color.
**c) KFe[Fe(CN)6]**
KFe[Fe(CN)6] is a coordination compound that contains both Fe(II) and Fe(III) ions. The Fe(II) ion has a +2 oxidation state, while the Fe(III) ion has a +3 oxidation state. The IVCT transitions in KFe[Fe(CN)6] involve the transfer of electrons between the Fe(II) and Fe(III) ions. These transitions can result in the absorption of light in the visible range, leading to the compound's characteristic yellow color.
**b) KCr2O7**
KCr2O7 is a compound that contains chromium (Cr) in the +6 oxidation state. While it does involve a difference in oxidation states, it does not contain two metal ions that can undergo IVCT transitions. Therefore, KCr2O7 does not exhibit IVCT transitions.
**d) Mn2(CO)10**
Mn2(CO)10 is a coordination compound that contains manganese (Mn) in the +2 oxidation state. It does not contain two metal ions with different oxidation states that can undergo IVCT transitions. Therefore, Mn2(CO)10 does not exhibit IVCT transitions.
In conclusion, the compounds that exhibit IVCT transitions are Pb3O4 (option A) and KFe[Fe(CN)6] (option C). These compounds contain metal ions with different oxidation states, allowing for the transfer of electrons and absorption of light in the visible range.
The compound which shows Metal to Ligand charge transfer is:- a)Ni(CO)4
- b)K2Cr2O7
- c)HgO
- d)[Ni(H2O)6]2+
Correct answer is option 'A'. Can you explain this answer?
The compound which shows Metal to Ligand charge transfer is:
a)
Ni(CO)4
b)
K2Cr2O7
c)
HgO
d)
[Ni(H2O)6]2+

|
Maitri Sen answered |
The compound that shows Metal to Ligand charge transfer is Ni(CO)4.
Explanation:
Metal to Ligand charge transfer refers to the transfer of an electron from a metal atom or ion to a ligand. This occurs when there is a difference in the electronegativity between the metal and the ligand, resulting in a polar bond.
In the given options, Ni(CO)4 is the only compound that shows Metal to Ligand charge transfer. Let's understand why:
1. Ni(CO)4:
- In this compound, nickel (Ni) is the metal atom and carbon monoxide (CO) is the ligand.
- Carbon monoxide is a strong sigma donor and a strong pi acceptor ligand. It donates its electron density to the metal through its sigma bond and accepts electron density from the metal through its pi* antibonding orbital.
- The difference in electronegativity between nickel and carbon is significant, leading to a polar bond.
- As a result, there is a charge transfer from the metal (Ni) to the ligand (CO), making it a Metal to Ligand charge transfer complex.
2. K2Cr2O7:
- In this compound, potassium (K) and chromium (Cr) are the metal atoms, and oxygen (O) is the ligand.
- Although there is a difference in electronegativity between the metals and the ligand, K2Cr2O7 does not show Metal to Ligand charge transfer. This is because the bonding in this compound is mainly ionic rather than covalent.
- The oxygen atoms in K2Cr2O7 act as bridging ligands, and there is no significant electron transfer between the metal and the ligand.
3. HgO:
- In this compound, mercury (Hg) is the metal atom, and oxygen (O) is the ligand.
- HgO does not show Metal to Ligand charge transfer because the bonding in this compound is mainly ionic in nature.
- The oxygen atom in HgO acts as an oxide ion, and there is no significant electron transfer between the metal and the ligand.
4. [Ni(H2O)6]2:
- In this compound, nickel (Ni) is the metal atom, and water (H2O) is the ligand.
- Although there is a difference in electronegativity between nickel and oxygen, [Ni(H2O)6]2 does not show Metal to Ligand charge transfer. This is because the bonding in this complex is mainly through coordination bonds, where the electron density is donated by the ligand to the metal, rather than vice versa.
In summary, among the given options, Ni(CO)4 is the only compound that shows Metal to Ligand charge transfer due to the significant difference in electronegativity between nickel and carbon monoxide.
Explanation:
Metal to Ligand charge transfer refers to the transfer of an electron from a metal atom or ion to a ligand. This occurs when there is a difference in the electronegativity between the metal and the ligand, resulting in a polar bond.
In the given options, Ni(CO)4 is the only compound that shows Metal to Ligand charge transfer. Let's understand why:
1. Ni(CO)4:
- In this compound, nickel (Ni) is the metal atom and carbon monoxide (CO) is the ligand.
- Carbon monoxide is a strong sigma donor and a strong pi acceptor ligand. It donates its electron density to the metal through its sigma bond and accepts electron density from the metal through its pi* antibonding orbital.
- The difference in electronegativity between nickel and carbon is significant, leading to a polar bond.
- As a result, there is a charge transfer from the metal (Ni) to the ligand (CO), making it a Metal to Ligand charge transfer complex.
2. K2Cr2O7:
- In this compound, potassium (K) and chromium (Cr) are the metal atoms, and oxygen (O) is the ligand.
- Although there is a difference in electronegativity between the metals and the ligand, K2Cr2O7 does not show Metal to Ligand charge transfer. This is because the bonding in this compound is mainly ionic rather than covalent.
- The oxygen atoms in K2Cr2O7 act as bridging ligands, and there is no significant electron transfer between the metal and the ligand.
3. HgO:
- In this compound, mercury (Hg) is the metal atom, and oxygen (O) is the ligand.
- HgO does not show Metal to Ligand charge transfer because the bonding in this compound is mainly ionic in nature.
- The oxygen atom in HgO acts as an oxide ion, and there is no significant electron transfer between the metal and the ligand.
4. [Ni(H2O)6]2:
- In this compound, nickel (Ni) is the metal atom, and water (H2O) is the ligand.
- Although there is a difference in electronegativity between nickel and oxygen, [Ni(H2O)6]2 does not show Metal to Ligand charge transfer. This is because the bonding in this complex is mainly through coordination bonds, where the electron density is donated by the ligand to the metal, rather than vice versa.
In summary, among the given options, Ni(CO)4 is the only compound that shows Metal to Ligand charge transfer due to the significant difference in electronegativity between nickel and carbon monoxide.
The bright yellow color of [Cu(phen)2]+ (phen=1, 10-phenanthroline) is due to- a)d-d transition
- b)metal to ligand chare transfer
- c)ligan to metal charge transfer
- d)π to π* transition in the phenanthroline ligand
Correct answer is option 'B'. Can you explain this answer?
The bright yellow color of [Cu(phen)2]+ (phen=1, 10-phenanthroline) is due to
a)
d-d transition
b)
metal to ligand chare transfer
c)
ligan to metal charge transfer
d)
π to π* transition in the phenanthroline ligand

|
Sinjini Nair answered |
Π-π* transition.
The violet color of iodine vapours is due to- a)n-π* transition
- b)π-π* transition
- c)n*-π* transition
- d)π*-σ* transition
Correct answer is option 'D'. Can you explain this answer?
The violet color of iodine vapours is due to
a)
n-π* transition
b)
π-π* transition
c)
n*-π* transition
d)
π*-σ* transition

|
Rishabh Mehta answered |
A) Electron transitions in the iodine molecule
Amongst the following, the strongest oxidizing anion is:- a)CrO42-
- b)VO43-
- c)FeO42-
- d)MnO42-
Correct answer is option 'D'. Can you explain this answer?
Amongst the following, the strongest oxidizing anion is:
a)
CrO42-
b)
VO43-
c)
FeO42-
d)
MnO42-

|
Aryan Gupta answered |
Strongest Oxidizing Anion
Oxidation is a chemical process in which a substance loses electrons and becomes positively charged. The oxidizing agent is the substance that accepts the electrons, while the reducing agent is the substance that donates the electrons. An oxidizing agent is a chemical species that can cause another substance to lose electrons. The strength of an oxidizing agent depends on its ability to accept electrons.
Among the given anions, the strongest oxidizing anion is MnO42-. This can be explained as follows:
- MnO42- contains manganese in its highest oxidation state of +7. This means that it has a high tendency to accept electrons and get reduced.
- MnO42- can easily accept electrons from other species and get reduced to MnO4-, MnO2 or even Mn2+ depending on the reaction conditions.
- The other anions, CrO42-, VO43-, and FeO42- contain chromium, vanadium, and iron in their highest oxidation states of +6, +5, and +6 respectively. These anions are also oxidizing agents but are less strong than MnO42-.
- The higher the oxidation state of the metal ion, the stronger is the oxidizing power of the anion.
Therefore, among the given anions, MnO42- is the strongest oxidizing anion.
Oxidation is a chemical process in which a substance loses electrons and becomes positively charged. The oxidizing agent is the substance that accepts the electrons, while the reducing agent is the substance that donates the electrons. An oxidizing agent is a chemical species that can cause another substance to lose electrons. The strength of an oxidizing agent depends on its ability to accept electrons.
Among the given anions, the strongest oxidizing anion is MnO42-. This can be explained as follows:
- MnO42- contains manganese in its highest oxidation state of +7. This means that it has a high tendency to accept electrons and get reduced.
- MnO42- can easily accept electrons from other species and get reduced to MnO4-, MnO2 or even Mn2+ depending on the reaction conditions.
- The other anions, CrO42-, VO43-, and FeO42- contain chromium, vanadium, and iron in their highest oxidation states of +6, +5, and +6 respectively. These anions are also oxidizing agents but are less strong than MnO42-.
- The higher the oxidation state of the metal ion, the stronger is the oxidizing power of the anion.
Therefore, among the given anions, MnO42- is the strongest oxidizing anion.
The pale yellow color of [Mn(H2O)6]2+ is due to- a)spin forbidden d-d transition
- b)metal to ligand charge transfer
- c)ligand to metal charge transfer
- d)intra ligand excitation
Correct answer is option 'A'. Can you explain this answer?
The pale yellow color of [Mn(H2O)6]2+ is due to
a)
spin forbidden d-d transition
b)
metal to ligand charge transfer
c)
ligand to metal charge transfer
d)
intra ligand excitation

|
Kunal Goyal answered |
Spin Forbidden d-d Transition
The pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This phenomenon occurs when there is a change in the electron configuration of the metal ion in a complex.
Explanation:
Spin Forbidden Transition:
When an electron in a transition metal complex moves from one d orbital to another, it involves a transition in the spin state of the electrons. According to the selection rules of electronic transitions, a change in spin from one state to another is considered spin forbidden. This means that the probability of such a transition occurring is very low.
Transition Metal Complexes:
Transition metal complexes are characterized by the presence of partially filled d orbitals in the central metal ion. These d orbitals can absorb light energy and undergo electronic transitions, resulting in the absorption or emission of light. The energy difference between the d orbitals determines the wavelength of light that is absorbed or emitted.
Pale Yellow Color of [Mn(H2O)6]2+:
In the case of [Mn(H2O)6]2+, the manganese ion (Mn2+) is in a high spin state with five unpaired electrons in its d orbitals. When light is absorbed by the complex, an electron is promoted from one d orbital to another. In this case, the d-d transition involves the promotion of an electron from a lower energy d orbital to a higher energy d orbital.
Spin Forbidden d-d Transition:
The pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This means that the transition between the d orbitals involves a change in the spin state of the electrons. According to the selection rules, a transition that involves a change in spin is unlikely to occur, leading to a low probability of absorption of light in this case.
Conclusion:
In summary, the pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This transition involves a change in the spin state of the electrons in the d orbitals of the manganese ion. As a result, the absorption of light is limited, leading to a pale yellow color observed for the complex.
The pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This phenomenon occurs when there is a change in the electron configuration of the metal ion in a complex.
Explanation:
Spin Forbidden Transition:
When an electron in a transition metal complex moves from one d orbital to another, it involves a transition in the spin state of the electrons. According to the selection rules of electronic transitions, a change in spin from one state to another is considered spin forbidden. This means that the probability of such a transition occurring is very low.
Transition Metal Complexes:
Transition metal complexes are characterized by the presence of partially filled d orbitals in the central metal ion. These d orbitals can absorb light energy and undergo electronic transitions, resulting in the absorption or emission of light. The energy difference between the d orbitals determines the wavelength of light that is absorbed or emitted.
Pale Yellow Color of [Mn(H2O)6]2+:
In the case of [Mn(H2O)6]2+, the manganese ion (Mn2+) is in a high spin state with five unpaired electrons in its d orbitals. When light is absorbed by the complex, an electron is promoted from one d orbital to another. In this case, the d-d transition involves the promotion of an electron from a lower energy d orbital to a higher energy d orbital.
Spin Forbidden d-d Transition:
The pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This means that the transition between the d orbitals involves a change in the spin state of the electrons. According to the selection rules, a transition that involves a change in spin is unlikely to occur, leading to a low probability of absorption of light in this case.
Conclusion:
In summary, the pale yellow color of [Mn(H2O)6]2+ is due to a spin forbidden d-d transition. This transition involves a change in the spin state of the electrons in the d orbitals of the manganese ion. As a result, the absorption of light is limited, leading to a pale yellow color observed for the complex.
The complex which exhibits lowest energy electronic absorption band is:- a)[NiCl4]2-
- b)[Ni(H2O)6]2+
- c)[Ni(CN)4]2-
- d)[Ni(CO)4]
Correct answer is option 'A'. Can you explain this answer?
The complex which exhibits lowest energy electronic absorption band is:
a)
[NiCl4]2-
b)
[Ni(H2O)6]2+
c)
[Ni(CN)4]2-
d)
[Ni(CO)4]

|
Jaya Sen answered |
Answer:
The complex which exhibits lowest energy electronic absorption band is [NiCl4]2-.
Explanation:
- The electronic absorption spectra of transition metal complexes arise due to the promotion of an electron from a lower energy d-orbital to a higher energy d-orbital or to the ligand field orbitals.
- The energy of the absorption band depends on the metal-ligand bond strength, the geometry of the complex, and the nature of the ligand field.
- Among the given options, [NiCl4]2- has the lowest energy absorption band because of the following reasons:
- It has a tetrahedral geometry with the nickel ion in the center and four chloride ions arranged symmetrically around it.
- The chloride ions are weak field ligands, which produce a small splitting of the d-orbitals of nickel.
- The energy required to promote an electron from the lower energy d-orbitals to the higher energy d-orbitals or to the ligand field orbitals is therefore relatively low.
- Hence, the absorption band is of low energy.
- The other options have the following characteristics:
- [Ni(H2O)6]2+ has an octahedral geometry with six water ligands. Water is a weak field ligand like chloride, and the energy of the absorption band is low but higher than [NiCl4]2-.
- [Ni(CN)4]2- has a square planar geometry with four cyanide ligands. Cyanide is a strong field ligand, which produces a large splitting of the d-orbitals of nickel. The energy required to promote an electron is therefore higher than the previous two options.
- [Ni(CO)4] has a tetrahedral geometry with four carbon monoxide ligands. Carbon monoxide is a strong field ligand, which produces a large splitting of the d-orbitals of nickel. The energy required to promote an electron is therefore the highest among the given options.
Therefore, the correct answer is option 'A' [NiCl4]2-.
The complex which exhibits lowest energy electronic absorption band is [NiCl4]2-.
Explanation:
- The electronic absorption spectra of transition metal complexes arise due to the promotion of an electron from a lower energy d-orbital to a higher energy d-orbital or to the ligand field orbitals.
- The energy of the absorption band depends on the metal-ligand bond strength, the geometry of the complex, and the nature of the ligand field.
- Among the given options, [NiCl4]2- has the lowest energy absorption band because of the following reasons:
- It has a tetrahedral geometry with the nickel ion in the center and four chloride ions arranged symmetrically around it.
- The chloride ions are weak field ligands, which produce a small splitting of the d-orbitals of nickel.
- The energy required to promote an electron from the lower energy d-orbitals to the higher energy d-orbitals or to the ligand field orbitals is therefore relatively low.
- Hence, the absorption band is of low energy.
- The other options have the following characteristics:
- [Ni(H2O)6]2+ has an octahedral geometry with six water ligands. Water is a weak field ligand like chloride, and the energy of the absorption band is low but higher than [NiCl4]2-.
- [Ni(CN)4]2- has a square planar geometry with four cyanide ligands. Cyanide is a strong field ligand, which produces a large splitting of the d-orbitals of nickel. The energy required to promote an electron is therefore higher than the previous two options.
- [Ni(CO)4] has a tetrahedral geometry with four carbon monoxide ligands. Carbon monoxide is a strong field ligand, which produces a large splitting of the d-orbitals of nickel. The energy required to promote an electron is therefore the highest among the given options.
Therefore, the correct answer is option 'A' [NiCl4]2-.
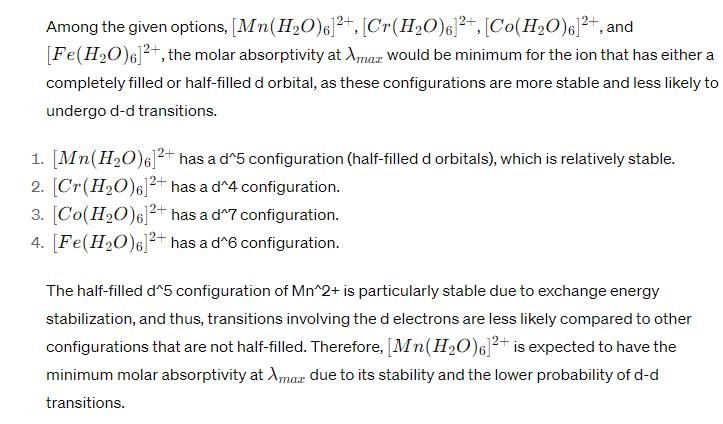
Which one of the following complex ions shows minimum intensity of absorption in the UV-Visible region?- a)[Cr(H2O)6]2+
- b)[V(H2O)6]2+
- c)[Mn(H2O)6]2+
- d)[Co(H2O)6]2+
Correct answer is option 'C'. Can you explain this answer?
Which one of the following complex ions shows minimum intensity of absorption in the UV-Visible region?
a)
[Cr(H2O)6]2+
b)
[V(H2O)6]2+
c)
[Mn(H2O)6]2+
d)
[Co(H2O)6]2+

|
Anshul Mehra answered |
UV-Visible Spectroscopy
UV-Visible spectroscopy is a technique used to study the absorption and transmission of light in the ultraviolet and visible regions of the electromagnetic spectrum. It is widely used in analytical chemistry and biochemistry to determine the concentration and purity of a sample.
Complex Ions
A complex ion is an ion in which a central metal ion is surrounded by one or more ligands. Ligands are molecules or ions that form a coordinate bond with the metal ion. The coordination number of a complex ion is the number of ligands attached to the metal ion.
Absorption Spectrum
The absorption spectrum of a compound is the pattern of absorption of light at different wavelengths. The absorption spectrum is obtained by passing light of different wavelengths through a sample and measuring the intensity of the light that is transmitted or absorbed.
Answer
The correct answer is option C, [Mn(H2O)6]2. This is because the absorption spectrum of [Mn(H2O)6]2 shows a minimum intensity of absorption in the UV-Visible region.
Explanation
The absorption spectrum of a complex ion depends on several factors, including the nature of the ligands, the coordination number of the metal ion, and the oxidation state of the metal ion.
In general, the absorption spectrum of a complex ion shows a series of peaks, known as bands, due to the electronic transitions between the metal ion and the ligands. The position, intensity, and shape of these bands depend on the energy difference between the metal ion and the ligands, as well as on the symmetry of the complex ion.
In the case of [Mn(H2O)6]2, the manganese ion has a coordination number of 6 and is surrounded by six water molecules as ligands. The absorption spectrum of [Mn(H2O)6]2 shows a weak band in the visible region, around 500-600 nm, and a weak band in the UV region, around 280-300 nm. However, the intensity of these bands is much lower than that of other transition metal complexes with similar coordination number and ligands.
This can be explained by the fact that the manganese ion in [Mn(H2O)6]2 has a d5 electronic configuration, which means that it has only one unpaired electron in its d orbitals. This makes the complex ion less stable and less able to absorb light than other transition metal complexes with a d6 or d7 electronic configuration.
Conclusion
In summary, [Mn(H2O)6]2 shows the minimum intensity of absorption in the UV-Visible region because of its d5 electronic configuration, which makes it less stable and less able to absorb light than other transition metal complexes with similar coordination number and ligands.
UV-Visible spectroscopy is a technique used to study the absorption and transmission of light in the ultraviolet and visible regions of the electromagnetic spectrum. It is widely used in analytical chemistry and biochemistry to determine the concentration and purity of a sample.
Complex Ions
A complex ion is an ion in which a central metal ion is surrounded by one or more ligands. Ligands are molecules or ions that form a coordinate bond with the metal ion. The coordination number of a complex ion is the number of ligands attached to the metal ion.
Absorption Spectrum
The absorption spectrum of a compound is the pattern of absorption of light at different wavelengths. The absorption spectrum is obtained by passing light of different wavelengths through a sample and measuring the intensity of the light that is transmitted or absorbed.
Answer
The correct answer is option C, [Mn(H2O)6]2. This is because the absorption spectrum of [Mn(H2O)6]2 shows a minimum intensity of absorption in the UV-Visible region.
Explanation
The absorption spectrum of a complex ion depends on several factors, including the nature of the ligands, the coordination number of the metal ion, and the oxidation state of the metal ion.
In general, the absorption spectrum of a complex ion shows a series of peaks, known as bands, due to the electronic transitions between the metal ion and the ligands. The position, intensity, and shape of these bands depend on the energy difference between the metal ion and the ligands, as well as on the symmetry of the complex ion.
In the case of [Mn(H2O)6]2, the manganese ion has a coordination number of 6 and is surrounded by six water molecules as ligands. The absorption spectrum of [Mn(H2O)6]2 shows a weak band in the visible region, around 500-600 nm, and a weak band in the UV region, around 280-300 nm. However, the intensity of these bands is much lower than that of other transition metal complexes with similar coordination number and ligands.
This can be explained by the fact that the manganese ion in [Mn(H2O)6]2 has a d5 electronic configuration, which means that it has only one unpaired electron in its d orbitals. This makes the complex ion less stable and less able to absorb light than other transition metal complexes with a d6 or d7 electronic configuration.
Conclusion
In summary, [Mn(H2O)6]2 shows the minimum intensity of absorption in the UV-Visible region because of its d5 electronic configuration, which makes it less stable and less able to absorb light than other transition metal complexes with similar coordination number and ligands.
The absorption of [Co(NH3)6]2+ is:- a)Stronger than that of [Co(NH3)5Cl]2+
- b)Stronger than that of [MnCl4]2-
- c)Weaker than that of [MnCl4]2-mbut stronger than that of [Co(NH3)5Cl]2+
- d)Weaker than those of [MnCl4]2- & [Co(NH3)5Cl]2+ both
Correct answer is option 'B'. Can you explain this answer?
The absorption of [Co(NH3)6]2+ is:
a)
Stronger than that of [Co(NH3)5Cl]2+
b)
Stronger than that of [MnCl4]2-
c)
Weaker than that of [MnCl4]2-mbut stronger than that of [Co(NH3)5Cl]2+
d)
Weaker than those of [MnCl4]2- & [Co(NH3)5Cl]2+ both

|
Shail Ghoshal answered |
The correct answer is c) Weaker than that of [MnCl4]2- but stronger than that of [Co(NH3)5Cl]2.
The absorption of a complex ion is determined by the nature of the ligands and the central metal ion. In this case, [Co(NH3)6]2- has six ammonia ligands, which are not as strong as chloride ligands in terms of their ability to form a coordination bond with the central metal ion. Therefore, the absorption of [Co(NH3)6]2- would be weaker than that of [MnCl4]2-.
On the other hand, [Co(NH3)5Cl]2- has five ammonia ligands and one chloride ligand. The presence of the chloride ligand makes the coordination bond stronger, resulting in a stronger absorption compared to [Co(NH3)6]2-. However, since [MnCl4]2- has four chloride ligands, it will have a stronger absorption than [Co(NH3)5Cl]2-.
Therefore, the correct answer is c) Weaker than that of [MnCl4]2- but stronger than that of [Co(NH3)5Cl]2.
The absorption of a complex ion is determined by the nature of the ligands and the central metal ion. In this case, [Co(NH3)6]2- has six ammonia ligands, which are not as strong as chloride ligands in terms of their ability to form a coordination bond with the central metal ion. Therefore, the absorption of [Co(NH3)6]2- would be weaker than that of [MnCl4]2-.
On the other hand, [Co(NH3)5Cl]2- has five ammonia ligands and one chloride ligand. The presence of the chloride ligand makes the coordination bond stronger, resulting in a stronger absorption compared to [Co(NH3)6]2-. However, since [MnCl4]2- has four chloride ligands, it will have a stronger absorption than [Co(NH3)5Cl]2-.
Therefore, the correct answer is c) Weaker than that of [MnCl4]2- but stronger than that of [Co(NH3)5Cl]2.
Which of the following transitions are of weak intensities and lie in the visible region?- a)n→n*
- b)σ→σ*
- c)π→π*
- d)n→σ*
Correct answer is option 'A'. Can you explain this answer?
Which of the following transitions are of weak intensities and lie in the visible region?
a)
n→n*
b)
σ→σ*
c)
π→π*
d)
n→σ*

|
Edurev.iitjam answered |
n→n* transitions are of weak intensities and lie in the visible region.
Chapter doubts & questions for Emission Spectra - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Emission Spectra - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup