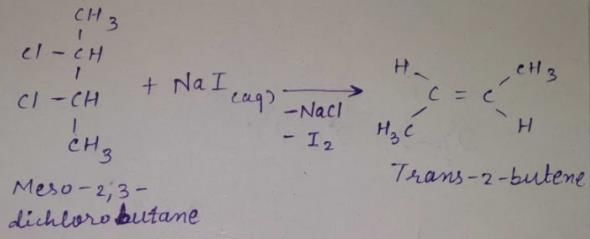All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Hydrocarbons and Nomenclature for EmSAT Achieve Exam
How many different isomeric alkynes on catalytic hydrogenation gives the same 3-ethyl hexane?- a)2
- b)3
- c)4
- d)6
Correct answer is option 'C'. Can you explain this answer?
How many different isomeric alkynes on catalytic hydrogenation gives the same 3-ethyl hexane?
a)
2
b)
3
c)
4
d)
6
|
|
Divya Menon answered |
Isomeric Alkynes and Catalytic Hydrogenation
Concept: Isomers are molecules that have the same molecular formula but different structural arrangements. Alkynes are hydrocarbons having at least one triple bond between two carbon atoms. Catalytic hydrogenation is the addition of hydrogen to an unsaturated organic compound in the presence of a catalyst. Catalytic hydrogenation of alkynes involves the addition of two hydrogen atoms across the triple bond, resulting in an alkane.
Explanation:
The given compound is 3-ethylhexane. The molecular formula of 3-ethylhexane is C8H18. The possible isomeric alkynes of C8H18 are:
1. Octyne-1
2. Octyne-2
3. Octyne-3
4. Octyne-4
5. Octyne-5
6. Octyne-6
Catalytic hydrogenation of octyne-1 and octyne-5 would result in the formation of the same product, i.e., 3-ethylhexane. Similarly, catalytic hydrogenation of octyne-2 and octyne-4 would also result in the formation of 3-ethylhexane. However, catalytic hydrogenation of octyne-3 and octyne-6 would result in the formation of different products.
Therefore, the total number of different isomeric alkynes that on catalytic hydrogenation gives the same 3-ethylhexane is 4.
Answer: The correct option is (C) 4.
Concept: Isomers are molecules that have the same molecular formula but different structural arrangements. Alkynes are hydrocarbons having at least one triple bond between two carbon atoms. Catalytic hydrogenation is the addition of hydrogen to an unsaturated organic compound in the presence of a catalyst. Catalytic hydrogenation of alkynes involves the addition of two hydrogen atoms across the triple bond, resulting in an alkane.
Explanation:
The given compound is 3-ethylhexane. The molecular formula of 3-ethylhexane is C8H18. The possible isomeric alkynes of C8H18 are:
1. Octyne-1
2. Octyne-2
3. Octyne-3
4. Octyne-4
5. Octyne-5
6. Octyne-6
Catalytic hydrogenation of octyne-1 and octyne-5 would result in the formation of the same product, i.e., 3-ethylhexane. Similarly, catalytic hydrogenation of octyne-2 and octyne-4 would also result in the formation of 3-ethylhexane. However, catalytic hydrogenation of octyne-3 and octyne-6 would result in the formation of different products.
Therefore, the total number of different isomeric alkynes that on catalytic hydrogenation gives the same 3-ethylhexane is 4.
Answer: The correct option is (C) 4.
Which of the following statements correctly describes the general reactivity of alkynes?- a)Alkynes are more reactive than alkenes
- b)Alkynes reacts as an electrophile
- c)Unlike alkenes, alkynes do not undergo electrophilic addition reaction
- d)An alkene is electron rich molecule, therefore react as a nucleophile
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements correctly describes the general reactivity of alkynes?
a)
Alkynes are more reactive than alkenes
b)
Alkynes reacts as an electrophile
c)
Unlike alkenes, alkynes do not undergo electrophilic addition reaction
d)
An alkene is electron rich molecule, therefore react as a nucleophile
|
|
Preeti Khanna answered |
The correct answer is option D
The clouds of electrons surrounding the sigma bond makes an alkyne an electron-rich molecule. They are therefore nucleophiles that react with electrophiles. Thus alkynes, like alkenes, undergo electrophilic addition reactions because of their weak pi bonds. When a proton adds to an alkyne, a vinylic cation is formed.
The clouds of electrons surrounding the sigma bond makes an alkyne an electron-rich molecule. They are therefore nucleophiles that react with electrophiles. Thus alkynes, like alkenes, undergo electrophilic addition reactions because of their weak pi bonds. When a proton adds to an alkyne, a vinylic cation is formed.
What is the major bromination product in the following reaction? 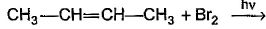
- a)
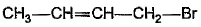
- b)
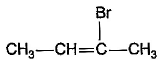
- c)
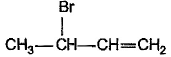
- d)
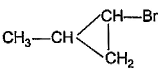
Correct answer is option 'C'. Can you explain this answer?
What is the major bromination product in the following reaction?
a)
b)
c)
d)

|
Mohit Rajpoot answered |
The correct answer is option c
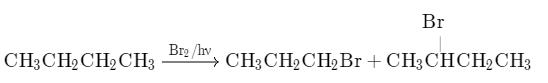
Thus, a racemic mixture is obtained. A racemic mixture is one that has an equal amount of left and right handed enantiomers of a chiral molecule.
Thus, a racemic mixture is obtained. A racemic mixture is one that has an equal amount of left and right handed enantiomers of a chiral molecule.
If 3-methyl-2-pentanol is heated with concentrated H2SO4 , in principle how many different alkenes result?
Correct answer is '5'. Can you explain this answer?
If 3-methyl-2-pentanol is heated with concentrated H2SO4 , in principle how many different alkenes result?
|
|
Krishna Iyer answered |
- methyl 1 pentene
- 3 methyl 2 butene
- 2 ethyl 1 butene
Hence 3 is the correct answer.
Propene on ozonolysis forms:- a)Acetaldehyde
- b)Formaldehyde
- c)Both acetaldehyde and formaldehyde
- d)Acetone
Correct answer is option 'C'. Can you explain this answer?
Propene on ozonolysis forms:
a)
Acetaldehyde
b)
Formaldehyde
c)
Both acetaldehyde and formaldehyde
d)
Acetone
|
|
Raghav Bansal answered |
When propene on ozonolysis it yields a new structure called ozonide
and there cleavage takes place and it yields two products namely
1.acetaldehyde
2.formaldehyde
and there cleavage takes place and it yields two products namely
1.acetaldehyde
2.formaldehyde
Direction (Q. Nos. 16 - 18) This section contains a paragraph, describing theory, experiments, data, etc.
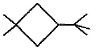
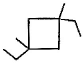
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (cj and (d).PassageA hydrocarbon with molecular formula C10H18, upon catalytic hydrogenation gives C10H20 (X). X on free radical chlorination gives two monochloro derivatives with their molecular formula C10H19CI that are constitutional isomers.Q. Which of the following satisfy the criteria of X ?- a)
- b)

- c)

- d)
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 16 - 18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (cj and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (cj and (d).
Passage
A hydrocarbon with molecular formula C10H18, upon catalytic hydrogenation gives C10H20 (X). X on free radical chlorination gives two monochloro derivatives with their molecular formula C10H19CI that are constitutional isomers.
Q. Which of the following satisfy the criteria of X ?
a)
b)
c)
d)
|
|
Krishna Iyer answered |
In option c, there are only two different position which can be chlorinated,
So, option c is correct.
So, option c is correct.
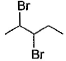
When vicinal dibromide is heated with KOH in ethanol (~ 200°C), double dehydrohalogenation takes place giving alkyne. Which of the following fails to give alkyne according to this procedure?- a)
- b)
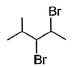
- c)
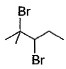
- d)
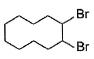
Correct answer is option 'C'. Can you explain this answer?
When vicinal dibromide is heated with KOH in ethanol (~ 200°C), double dehydrohalogenation takes place giving alkyne. Which of the following fails to give alkyne according to this procedure?
a)
b)
c)
d)
|
|
Rajesh Gupta answered |
The correct answer is option C
CH³)²BrC*–CHBr–CH²–CH³
C* has no H attached to it hence the next Bromine cannot perform dehydrohalogenation using this Carbon. Therefore, triple bond formation is not possible in this compound.
CH³)²BrC*–CHBr–CH²–CH³
C* has no H attached to it hence the next Bromine cannot perform dehydrohalogenation using this Carbon. Therefore, triple bond formation is not possible in this compound.
How many different heptenes result by partial hydrogenation of all possible, unbranched heptynes?
Correct answer is '5'. Can you explain this answer?
How many different heptenes result by partial hydrogenation of all possible, unbranched heptynes?
|
|
Neha Joshi answered |
The correct answer is 5.
C=C-C-C-C-C-C , no geometrical isomerism so 1
C-C=C-C-C-C-C 2 , geometrical cis trans
C-C-C=C-C-C-C , cis trans again so 2
∴ 2+2+1=5
C=C-C-C-C-C-C , no geometrical isomerism so 1
C-C=C-C-C-C-C 2 , geometrical cis trans
C-C-C=C-C-C-C , cis trans again so 2
∴ 2+2+1=5
Out of the following compounds , which will be have a zero dipole moment.- a)1,1 - dichloroethylene
- b)cis-1,2-dichlorethylene
- c)trans-1,2-dichlorethylene
- d)none of these.
Correct answer is option 'C'. Can you explain this answer?
Out of the following compounds , which will be have a zero dipole moment.
a)
1,1 - dichloroethylene
b)
cis-1,2-dichlorethylene
c)
trans-1,2-dichlorethylene
d)
none of these.
|
|
Hansa Sharma answered |
In case of trans-1,2-dichloroethylene
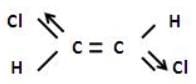
The net dipole moment is zero but in case of cis -1,2 -dicholorethylene.
There is some resultant dipole moment.
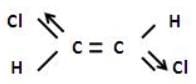
The net dipole moment is zero but in case of cis -1,2 -dicholorethylene.
There is some resultant dipole moment.
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many different alkenes are formed when 2 -chlorobutane is treated with ethanolic solution of KOH?
- a)1
- b)3
- c)2
- d)4
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many different alkenes are formed when 2 -chlorobutane is treated with ethanolic solution of KOH?
a)
1
b)
3
c)
2
d)
4
|
|
Arya Dasgupta answered |
The reaction of 2-chlorobutane with ethanolic solution of KOH is an example of an elimination reaction known as dehydrohalogenation. In this reaction, a hydrogen atom and a halogen atom are removed from adjacent carbon atoms to form a double bond.
The possible alkenes that can be formed are:
1. Butene
2. But-2-ene
3. But-1-ene
4. Isobutene
The number of different alkenes formed depends on the position of the double bond. In the case of 2-chlorobutane, the chlorine atom is attached to the second carbon atom, so the double bond can form between the first and second carbon atoms or between the second and third carbon atoms.
Hence, the correct answer is option (b) 2.
The possible alkenes that can be formed are:
1. Butene
2. But-2-ene
3. But-1-ene
4. Isobutene
The number of different alkenes formed depends on the position of the double bond. In the case of 2-chlorobutane, the chlorine atom is attached to the second carbon atom, so the double bond can form between the first and second carbon atoms or between the second and third carbon atoms.
Hence, the correct answer is option (b) 2.
Direction (Q, Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.Q.Statement I : 2-butyne on reduction with Pd/CaCO3 gives c/s-2-butene.
Statement II : Hydrogenation proceed through adsorption mechanism.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Direction (Q, Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : 2-butyne on reduction with Pd/CaCO3 gives c/s-2-butene.
Statement II : Hydrogenation proceed through adsorption mechanism.
Statement II : Hydrogenation proceed through adsorption mechanism.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Raghav Bansal answered |
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulphate which is then poisoned with various forms of lead or sulphur. It is used for the hydrogenation of alkynes to cis alkenes (i.e. without further reduction into alkanes) When the triple bond is (2-butyne) hydrogenated over the Lindlar’s catalyst i.e.Pd/CaCO3
it gives predominantly cis alkene(2-butene).
it gives predominantly cis alkene(2-butene).
Ethylene reacts with HBr to give:- a)Acetylene
- b)Ethyl alcohol
- c)Acetaldehyde
- d)Ethyl bromide
Correct answer is option 'D'. Can you explain this answer?
Ethylene reacts with HBr to give:
a)
Acetylene
b)
Ethyl alcohol
c)
Acetaldehyde
d)
Ethyl bromide
|
|
Preeti Iyer answered |
Ethylene reacts with HBr to form Ethyl bromide. The reaction propagates as follow:-
H2C=CH2 + HBr → H2C+-CH3 →H2BrC-CH3
Since π cloud is electron rich, so HBr dissociates into H+ and Br-. H+ attacks on alkene to give a carbocation and then Br- attacks to get ethyl bromide.
H2C=CH2 + HBr → H2C+-CH3 →H2BrC-CH3
Since π cloud is electron rich, so HBr dissociates into H+ and Br-. H+ attacks on alkene to give a carbocation and then Br- attacks to get ethyl bromide.
Ethene and ethyne can be distinguished by:- a)Bromine water
- b)KMnO4 solution
- c)Ammoniacal Cuprous chloride solution
- d)Any of the above
Correct answer is option 'C'. Can you explain this answer?
Ethene and ethyne can be distinguished by:
a)
Bromine water
b)
KMnO4 solution
c)
Ammoniacal Cuprous chloride solution
d)
Any of the above
|
|
Nandini Patel answered |
The two hydrocarbons can be easily distinguished by simple chemical tests, as ethyne molecule is supposed to have acidic hydrogen.
1. When ethyne is bubbled through ammoniacal silver nitrate solution , a yellow-white precipitate of silver acetylide would be formed.
C2H2 + 2AgNO3 = Ag2C2 + 2HNO3
2. Similarly, ethyne forms a red precipitate of copper acetylide (Cu2C2) when it is passed through ammoniacal cuprous chloride solution.
Ethene does not react with AgNO3 or Cu2Cl2 solution.
Direction (Q. Nos. 12 -15) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. In the following free radical bromination reaction, the im portant product(s) is/are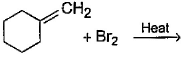
- a)
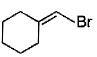
- b)
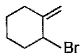
- c)
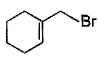
- d)
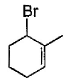
Correct answer is option 'B,C'. Can you explain this answer?
Direction (Q. Nos. 12 -15) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. In the following free radical bromination reaction, the im portant product(s) is/are
a)
b)
c)
d)
|
|
Lavanya Menon answered |
This is the case of allylic substitution. The radical will delocalise itself to two locations. And so, we will get two different positions for radical substitution.
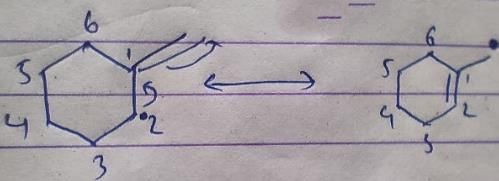
There is also a possibility at C6, but it is not in our option.

There is also a possibility at C6, but it is not in our option.
Direction (Q. Nos. 17 - 20) This section contains 4 questions. When worked o u t wili result in an integer from 0 to 9 (both inclusive).Q. If 3-bromo-4-methyl hexane is treated with ethanolic KOH solution, how many different alkenes would be formed?
Correct answer is '6'. Can you explain this answer?
Direction (Q. Nos. 17 - 20) This section contains 4 questions. When worked o u t wili result in an integer from 0 to 9 (both inclusive).
Q. If 3-bromo-4-methyl hexane is treated with ethanolic KOH solution, how many different alkenes would be formed?
|
|
Raghav Bansal answered |
2 is the right answer.
Alcoholic KOH causes dehydrohalogenation reaction i.e. elimination reaction.
Which of the following reactions can bring about chlorination of cyclohexane?- a)
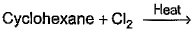
- b)
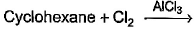
- c)
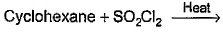
- d)
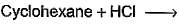
Correct answer is option 'A,C'. Can you explain this answer?
Which of the following reactions can bring about chlorination of cyclohexane?
a)
b)
c)
d)

|
Knowledge Hub answered |
For SO2Cl2: The reactivity patterns of SO2Cl2 and SOCl2 are quite different. SOCl2 is a good electrophile, and can be thought of as a source of Cl− ions. These ions can go on to react in their typical nucleophilic fashion. SO2Cl2 however is often a Cl2 source, as it readily decomposes giving off sulfur dioxide. Usually, much easier/safer to use this than measuring out (and getting into solution) chlorine gas. The chlorination of simple alkanes by Cl2 gas (or something that makes it in solution) happens by a radical mechanism i.e. Cl⋅ not Cl
For Cl2 and heat/light:
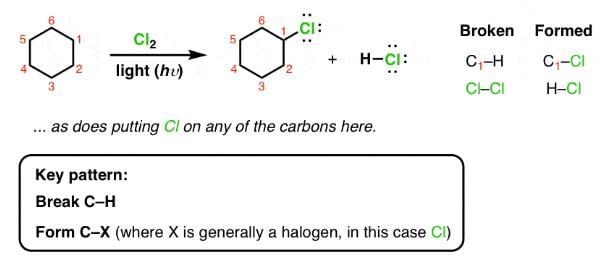
For Cl2 and heat/light:

For Cl with AlCl3: It is used for chlorination of compounds like benzene
For HCl: It is used for halogenations of a double bond.
For HCl: It is used for halogenations of a double bond.
Cis isomer have:- a)High boiling point than trans isomer
- b)Lower boiling point than trans isomer
- c)Same boiling point
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Cis isomer have:
a)
High boiling point than trans isomer
b)
Lower boiling point than trans isomer
c)
Same boiling point
d)
None of the above
|
|
Rajat Kapoor answered |
The cis isomer in this case has a boiling point of 60.3 degC, while the trans isomer has a boiling point of 47.5 degC. In the cis isomer the two polar C-Cl bond dipole moments combine to give an overall molecular dipole, so that there are intermolecular dipole–dipole forces (or Keesom forces), which add to the London.
Which among these is not a structural isomer of the compound C4H8?- a)But-1-ene
- b)But-2-ene
- c)But-3-ene
- d)2-methylpropene
Correct answer is option 'C'. Can you explain this answer?
Which among these is not a structural isomer of the compound C4H8?
a)
But-1-ene
b)
But-2-ene
c)
But-3-ene
d)
2-methylpropene

|
Saqib Shabir answered |
The answer is c. But-3-ene. But-3-ene is not a structural isomer of C4H8 because it is the same molecule as But-1-ene, just numbered differently. The structural isomers of C4H8 are But-1-ene, But-2-ene, and 2-methylpropene. But-1-ene and But-2-ene are position isomers, differing in the position of the double bond. 2-methylpropene is a branched isomer. But-3-ene is not a distinct isomer because the numbering of the carbon chain starts from the end closest to the double bond, making it identical to But-1-ene.
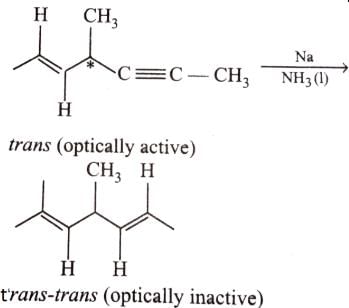
The correct statement(s) regarding 1,2-butadiene(l) and 1,3-butadiene, (II) is/are- a)I has perpendicular pi planes while II has parallel pi planes
- b)Both gives the same product on adding 1.0 equivalent at HBr of -80° C
- c)I gives 2, 2-dibromobutane while II gives 2, 3-dibromobutane, when reacted with excess of HBr at very low temperature
- d)Both have same length of C = C double bonds
Correct answer is option 'A,C'. Can you explain this answer?
The correct statement(s) regarding 1,2-butadiene(l) and 1,3-butadiene, (II) is/are
a)
I has perpendicular pi planes while II has parallel pi planes
b)
Both gives the same product on adding 1.0 equivalent at HBr of -80° C
c)
I gives 2, 2-dibromobutane while II gives 2, 3-dibromobutane, when reacted with excess of HBr at very low temperature
d)
Both have same length of C = C double bonds
|
|
Vivek answered |
Adjacent hybrid orbitals are perpendicular to each other irrespective of the type of the hybridization. In 1,2-butadiene, adjacent 2× bonds are perpendicular to each other while in 1,3-butadiene, 2× bond and
1× bond are adjacent to each other and the compound is a straight chain, so, the single bone and a double bond are perpendicular to each other and this is true for both the pairs of single and double bonds. Thus , both the pi orbitals in 1,3-butadiene are parallel to each other.
I hope it helps. If u still have any doubt, u can discuss with me.
1× bond are adjacent to each other and the compound is a straight chain, so, the single bone and a double bond are perpendicular to each other and this is true for both the pairs of single and double bonds. Thus , both the pi orbitals in 1,3-butadiene are parallel to each other.
I hope it helps. If u still have any doubt, u can discuss with me.
Consider the following reaction sequence. The correct statement(s) regarding diois formed in the final step is/are
The correct statement(s) regarding diois formed in the final step is/are - a)Three different diois are formed
- b)One pair of diastereomers is formed
- c)Two enantiomers are formed
- d)Product is optically inactive
Correct answer is option 'A,C'. Can you explain this answer?
Consider the following reaction sequence.
The correct statement(s) regarding diois formed in the final step is/are
a)
Three different diois are formed
b)
One pair of diastereomers is formed
c)
Two enantiomers are formed
d)
Product is optically inactive
|
|
Om Desai answered |
The correct answers are Options A and C.
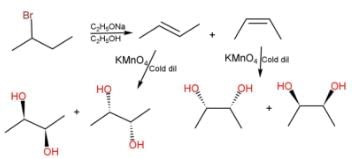

Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. How many distinct alkynes exist for C6H10 which gives effervescence on heating with Na?- a)5
- b)2
- c)3
- d)4
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. How many distinct alkynes exist for C6H10 which gives effervescence on heating with Na?
a)
5
b)
2
c)
3
d)
4
|
|
Upasana Bose answered |
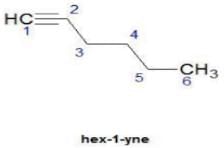
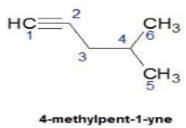
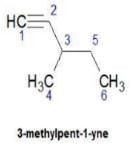
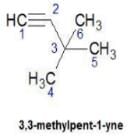
Hence D
Which of the following is not a possible termination step in the free radical chlorination of methane?- a)
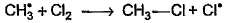
- b)

- c)
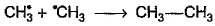
- d)
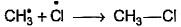
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a possible termination step in the free radical chlorination of methane?
a)
b)
c)
d)

|
Lohit Matani answered |
Termination is the last step. So there shouldn't be any free radical atom remaining. In first option there is Cl• remaining it can't be termination step.The steps in free radical halogenation are as
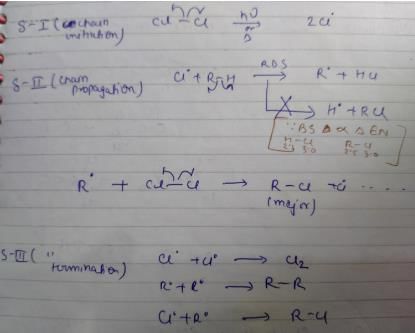
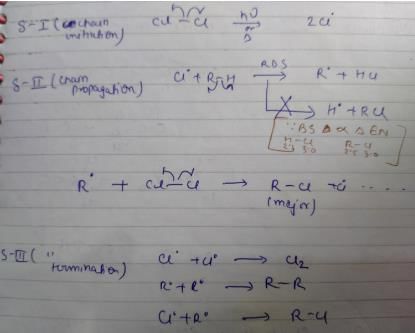
Direction (Q. Nos, 19 - 22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Q. How many different isomers of alkenes {including stereoisomers) exist that all upon catalytic hydrogenation adds one mole of H2 to give the same 2, 2, 3,5-tetramethyl hexane?
Correct answer is '7'. Can you explain this answer?
Direction (Q. Nos, 19 - 22) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. How many different isomers of alkenes {including stereoisomers) exist that all upon catalytic hydrogenation adds one mole of H2 to give the same 2, 2, 3,5-tetramethyl hexane?

|
Ashish Mishra answered |
7 is correct.
If 4-methyl cyclopentene is treated with OsO4 followed by work-up with NaHSO3/H2O, how many different diols would result?
Correct answer is '2'. Can you explain this answer?
If 4-methyl cyclopentene is treated with OsO4 followed by work-up with NaHSO3/H2O, how many different diols would result?
|
|
Preeti Iyer answered |
Since, OsO4 + NaHSO4/H2O syn hydroxylation, so two products can be obtained.
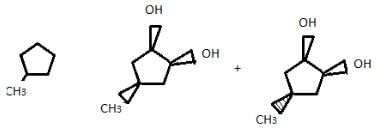

Addition of halogens to alkenes is an example of:- a)Nucleophilic addition reaction
- b)Electrophilic addition reaction
- c)Electrophilic substitution reaction
- d)Nucleophilic substitution reaction
Correct answer is option 'B'. Can you explain this answer?
Addition of halogens to alkenes is an example of:
a)
Nucleophilic addition reaction
b)
Electrophilic addition reaction
c)
Electrophilic substitution reaction
d)
Nucleophilic substitution reaction

|
Avantika Chakraborty answered |
Halogens can act as electrophiles to attack a double bond in alkene. Double bond represents a region of electron density and therefore functions as a nucleophile.
A hydrocarbon with molecular formula C10H18, upon catalytic hydrogenation gives C10H20 (X). X on free radical chlorination gives two monochloro derivatives with their molecular formula C10H19CI that are constitutional isomers.Q. How many different alkenes on hydrogenation, can gives X ?- a)1
- b)2
- c)3
- d)4
Correct answer is option 'A'. Can you explain this answer?
A hydrocarbon with molecular formula C10H18, upon catalytic hydrogenation gives C10H20 (X). X on free radical chlorination gives two monochloro derivatives with their molecular formula C10H19CI that are constitutional isomers.
Q. How many different alkenes on hydrogenation, can gives X ?
a)
1
b)
2
c)
3
d)
4
|
|
Gaurav Kumar answered |
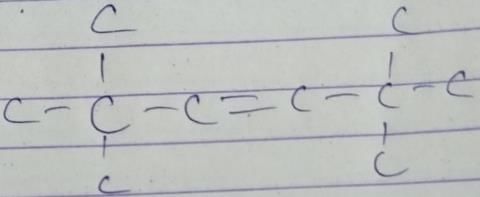
Direction (Q. Nos. 1 - 11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. When light is shined on a mixture of chlorine and ethane, chloroethane is formed besides dichloroethane, trichloroethane and several other products. What reaction condition can optimise the yield of chloroethane?- a)Higher reaction temperature
- b)High concentration of chlorine gas
- c)Excess of ethane reactan
- d)Low reaction temperature
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1 - 11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. When light is shined on a mixture of chlorine and ethane, chloroethane is formed besides dichloroethane, trichloroethane and several other products. What reaction condition can optimise the yield of chloroethane?
a)
Higher reaction temperature
b)
High concentration of chlorine gas
c)
Excess of ethane reactan
d)
Low reaction temperature
|
|
Pooja Shah answered |
When chlorine and Ethane are taken with chlorine in excess only then we have more than one product like chloroethane, dichloroethane, trichloroethane etc. To avoid this we should take Ethane in excess because when we will take it then in excess then we will have only single time chlorination and we will get monochloroethane.
Can you explain the answer of this question below:Consider the following transformations.
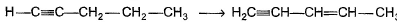
Q. Which reaction sequence will bent bring about the above transformation?
- A:
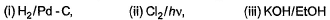
- B:
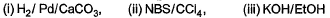
- C:
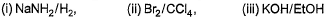
- D:
The answer is b.
Consider the following transformations.
Q. Which reaction sequence will bent bring about the above transformation?
|
|
Hansa Sharma answered |
we get H-CH=C-CH2-CH2-CH3
H-CH=C-CH2-CH2-CH3 + NBS/CCl4 → H-CH=C-CHCl-CH2-CH3 (NBS adds Br to carbon at next alternate to double bond)
H-CH=C-CHCl-CH2-CH3 + KOH/EtOH → H2C=C-CH=CH-CH3
(by E2 elimination)
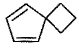
What is the major dehydration product in the following reaction ?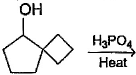
- a)

- b)

- c)
- d)

Correct answer is option 'D'. Can you explain this answer?
What is the major dehydration product in the following reaction ?
a)
b)
c)
d)
|
|
Pooja Shah answered |
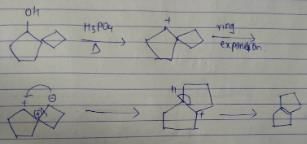
Note that H2SO4, H3PO4 and KHSO4 are dehydrating agents with rearrangement possible. While P2O5, P4O10, ThO2, POCl3 and Al2O3 are dehydrating agents without any possibility of rearrangement.
During free radical bromination of isobutane, an alkene is produced as by product via disproportionation of the intermediate alkyl free radical. What is this alkene?- a)1-butene
- b)2-butene
- c)2-methyl propene
- d)Both ‘b’ and ‘c’
Correct answer is option 'C'. Can you explain this answer?
During free radical bromination of isobutane, an alkene is produced as by product via disproportionation of the intermediate alkyl free radical. What is this alkene?
a)
1-butene
b)
2-butene
c)
2-methyl propene
d)
Both ‘b’ and ‘c’
|
|
Ishani Pillai answered |
The alkene produced as a byproduct during the free radical bromination of isobutane is 2-methyl propene (option c). Let's understand the process in detail:
1. Free Radical Bromination:
During free radical bromination, isobutane (C4H10) reacts with a bromine radical (Br•) to form a primary alkyl radical (C4H9•). This reaction is initiated by the presence of light or heat.
C4H10 + Br• → C4H9• + HBr
2. Disproportionation of the Intermediate Alkyl Free Radical:
The intermediate alkyl free radical (C4H9•) can undergo different reactions, including abstraction of a hydrogen atom from another isobutane molecule or rearrangement. However, in this case, it undergoes disproportionation to form a new alkene.
C4H9• → Alkene + Alkane
3. Formation of 2-Methyl Propene:
The disproportionation of the C4H9• radical leads to the formation of 2-methyl propene. This alkene is obtained when the alkyl radical undergoes a β-scission, resulting in the formation of a double bond.
C4H9• → 2-methyl propene + Alkane
Hence, during the free radical bromination of isobutane, 2-methyl propene is produced as a byproduct through the disproportionation of the intermediate alkyl free radical.
To summarize:
- Isobutane reacts with a bromine radical to form a primary alkyl radical.
- The primary alkyl radical can undergo various reactions, but in this case, it undergoes disproportionation.
- The disproportionation of the alkyl radical leads to the formation of 2-methyl propene as a byproduct.
1. Free Radical Bromination:
During free radical bromination, isobutane (C4H10) reacts with a bromine radical (Br•) to form a primary alkyl radical (C4H9•). This reaction is initiated by the presence of light or heat.
C4H10 + Br• → C4H9• + HBr
2. Disproportionation of the Intermediate Alkyl Free Radical:
The intermediate alkyl free radical (C4H9•) can undergo different reactions, including abstraction of a hydrogen atom from another isobutane molecule or rearrangement. However, in this case, it undergoes disproportionation to form a new alkene.
C4H9• → Alkene + Alkane
3. Formation of 2-Methyl Propene:
The disproportionation of the C4H9• radical leads to the formation of 2-methyl propene. This alkene is obtained when the alkyl radical undergoes a β-scission, resulting in the formation of a double bond.
C4H9• → 2-methyl propene + Alkane
Hence, during the free radical bromination of isobutane, 2-methyl propene is produced as a byproduct through the disproportionation of the intermediate alkyl free radical.
To summarize:
- Isobutane reacts with a bromine radical to form a primary alkyl radical.
- The primary alkyl radical can undergo various reactions, but in this case, it undergoes disproportionation.
- The disproportionation of the alkyl radical leads to the formation of 2-methyl propene as a byproduct.
The alkene which on ozonolysis gives only acetone is:- a)(CH3)2C = CH2
- b)CH3CH = CHCH3
- c)CH3CH = C(CH3)2
- d)(CH3)2C = C(CH3)2
Correct answer is option 'D'. Can you explain this answer?
The alkene which on ozonolysis gives only acetone is:
a)
(CH3)2C = CH2
b)
CH3CH = CHCH3
c)
CH3CH = C(CH3)2
d)
(CH3)2C = C(CH3)2

|
Pioneer Academy answered |
The reductive ozaonalysis of 2,3 -Dimethyl-2-butene yields acetone.The reaction is as follows: 2,3 -Dimethyl-2-butene acetone
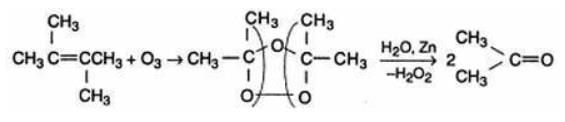
Which of the following statem ents regarding free radical halogenation o f alkane is not true?- a)Hydrogen abstraction by halogen radical in the propagation step is exothermic in both chlorination and bromination
- b)Hydrogen abstraction by halogen radical in propagation step is exothermic in chlorination but endothermic in bromination
- c)A single halogen radical may bring about halogenation of thousands of alkane molecules
- d)Continuous source of energy is required to continue the free radical halogenation reaction
Correct answer is option 'A'. Can you explain this answer?
Which of the following statem ents regarding free radical halogenation o f alkane is not true?
a)
Hydrogen abstraction by halogen radical in the propagation step is exothermic in both chlorination and bromination
b)
Hydrogen abstraction by halogen radical in propagation step is exothermic in chlorination but endothermic in bromination
c)
A single halogen radical may bring about halogenation of thousands of alkane molecules
d)
Continuous source of energy is required to continue the free radical halogenation reaction
|
|
Geetika Shah answered |
Hydrogen abstraction by halogen radical in the propagation step is exothermic in chlorination but endothermic in bromination. Hence, the option (A) is an incorrect statement.
An optically active hydrocarbon X has molecular formula C6H12. X on catalytic hydrogenation gives optically inactive C6H14. X could be- a)3-methyl-1-pentene
- b)3-methyl-2-pentene
- c)4-methyl-2-pentene
- d)2-ethyl-1 -butene
Correct answer is option 'A'. Can you explain this answer?
An optically active hydrocarbon X has molecular formula C6H12. X on catalytic hydrogenation gives optically inactive C6H14. X could be
a)
3-methyl-1-pentene
b)
3-methyl-2-pentene
c)
4-methyl-2-pentene
d)
2-ethyl-1 -butene
|
|
Pritam Kapoor answered |
The optically active C6H1 2 hydrocarbon is 3-Methylpent-1-ene, having one chiral carbon shown in fig A.
On catalytic hydrogenation , the compound obtained is shown in fig B, and the molecule does not have chiral carbon.
The reaction is C6H1 2 → C6H1 4 and this reaction takes place inthe presence of H2 and Pd.
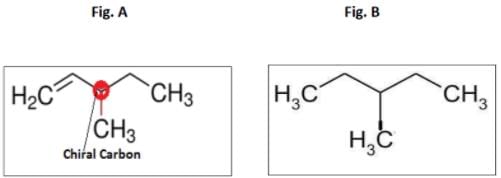
On catalytic hydrogenation , the compound obtained is shown in fig B, and the molecule does not have chiral carbon.
The reaction is C6H1 2 → C6H1 4 and this reaction takes place inthe presence of H2 and Pd.

In which of the following reactions , only single is omer of alkene is formed ?- a)
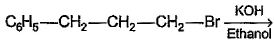
- b)
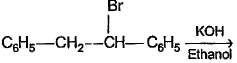
- c)
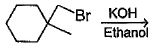
- d)

Correct answer is option 'A'. Can you explain this answer?
In which of the following reactions , only single is omer of alkene is formed ?
a)
b)
c)
d)
|
|
Pooja Shah answered |
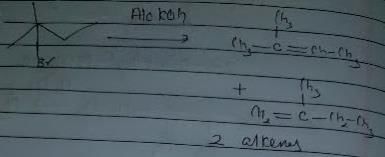
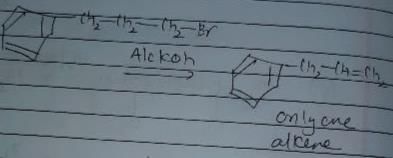
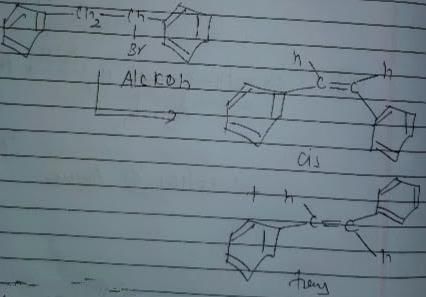
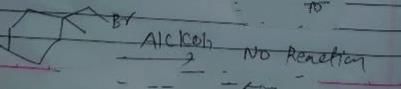
As there is no hydrogen at adjacent position for E2 elimination.
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?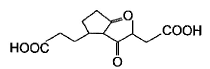
- a)
- b)
- c)
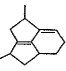
- d)
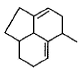
Correct answer is option 'D'. Can you explain this answer?
Direction (Q, Nos. 1 - 5) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which molecule will give the following dicarboxylic acid upon treatment with acidic solution of KMnO4?
a)
b)
c)
d)
|
|
Om Desai answered |
The correct answer is Option D.


Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
- a)IV < III < II < I
- b)I < II < IV < III
- c)I < II < III < IV
- d)II < I < IV < III
Correct answer is option 'C'. Can you explain this answer?
Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
a)
IV < III < II < I
b)
I < II < IV < III
c)
I < II < III < IV
d)
II < I < IV < III
|
|
Om Desai answered |
For boiling point, we have to consider both branching and Molecular mass. In 4 bromopentane molecular mass is nearly the same as compared to 3 chloro pentane but we have 3,3-dichloropentane extended into 2 directions so the boiling point of 3,3-dichloropentane will be more and the other order will be followed by option C.
When H+ attacks CH3 – CH = CH2 , carbonation which is more stable is- a)CH3 – CH2 – CH2
- b)CH2+ – CH2 – CH3
- c)CH3 – CH+ – CH2
- d)CH3 – CH2 – CH2+
Correct answer is option 'C'. Can you explain this answer?
When H+ attacks CH3 – CH = CH2 , carbonation which is more stable is
a)
CH3 – CH2 – CH2
b)
CH2+ – CH2 – CH3
c)
CH3 – CH+ – CH2
d)
CH3 – CH2 – CH2+
|
|
Nitin Khanna answered |
CH3 – CH = CH2 → CH3 – CH+ – CH2
The reason for this is only that carbocation is formed which has maximum stability. In this case, we have 6 α-H while for option a, b and d; we have 0, 2 and 2 α-H respectively. So only carbocation in option c forms.
The reason for this is only that carbocation is formed which has maximum stability. In this case, we have 6 α-H while for option a, b and d; we have 0, 2 and 2 α-H respectively. So only carbocation in option c forms.
Which of the following compounds react most readily with Br2(g)?
- a) C2H2
- b) C3H6
- c)C2H4
- d)C4H10
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds react most readily with Br2(g)?
a)
C2H2
b)
C3H6
c)
C2H4
d)
C4H10
|
|
Siddharth Iyer answered |
The compound that reacts most readily with Br(g) is C3H6. Here's why:
Explanation:
When a compound reacts with Br(g), it undergoes a substitution reaction called bromination. In this reaction, a Br atom replaces a hydrogen atom in the compound. The reactivity of a compound towards bromination depends on its structure and the stability of the resulting product.
Comparing the compounds:
Let's compare the given compounds and analyze their structures to determine which one is most reactive towards bromination.
a) C2H2:
C2H2 is an alkyne with a triple bond between two carbon atoms. This triple bond is very strong and stable, making it difficult for Br(g) to break it and substitute a hydrogen atom. Therefore, C2H2 is less reactive towards bromination.
b) C3H6:
C3H6 is an alkene with a double bond between two carbon atoms. The double bond is weaker and less stable than a triple bond. Therefore, it is easier for Br(g) to break the double bond and substitute a hydrogen atom. This makes C3H6 more reactive towards bromination compared to C2H2.
c) C2H4:
C2H4 is also an alkene with a double bond between two carbon atoms, similar to C3H6. It has the same structure as C3H6, but it has fewer carbon atoms. Since the number of carbon atoms does not significantly affect the reactivity towards bromination, C2H4 is also reactive towards bromination, but less reactive compared to C3H6.
d) C4H10:
C4H10 is an alkane with only single bonds between carbon atoms. Alkanes are generally less reactive towards bromination because the single bonds are strong and stable. Breaking a single bond to substitute a hydrogen atom is more difficult for Br(g) compared to breaking a double or triple bond. Therefore, C4H10 is the least reactive towards bromination among the given compounds.
Conclusion:
Based on the structural analysis and the stability of the bonds, C3H6 is the most reactive compound towards bromination among the given options.
Explanation:
When a compound reacts with Br(g), it undergoes a substitution reaction called bromination. In this reaction, a Br atom replaces a hydrogen atom in the compound. The reactivity of a compound towards bromination depends on its structure and the stability of the resulting product.
Comparing the compounds:
Let's compare the given compounds and analyze their structures to determine which one is most reactive towards bromination.
a) C2H2:
C2H2 is an alkyne with a triple bond between two carbon atoms. This triple bond is very strong and stable, making it difficult for Br(g) to break it and substitute a hydrogen atom. Therefore, C2H2 is less reactive towards bromination.
b) C3H6:
C3H6 is an alkene with a double bond between two carbon atoms. The double bond is weaker and less stable than a triple bond. Therefore, it is easier for Br(g) to break the double bond and substitute a hydrogen atom. This makes C3H6 more reactive towards bromination compared to C2H2.
c) C2H4:
C2H4 is also an alkene with a double bond between two carbon atoms, similar to C3H6. It has the same structure as C3H6, but it has fewer carbon atoms. Since the number of carbon atoms does not significantly affect the reactivity towards bromination, C2H4 is also reactive towards bromination, but less reactive compared to C3H6.
d) C4H10:
C4H10 is an alkane with only single bonds between carbon atoms. Alkanes are generally less reactive towards bromination because the single bonds are strong and stable. Breaking a single bond to substitute a hydrogen atom is more difficult for Br(g) compared to breaking a double or triple bond. Therefore, C4H10 is the least reactive towards bromination among the given compounds.
Conclusion:
Based on the structural analysis and the stability of the bonds, C3H6 is the most reactive compound towards bromination among the given options.
Passage IIAn organic compound X (C7H11Br) shows optical isomerism as well as decolourises brown colour of bromine water solution. X on treatment with HBr in the absence of a peroxide forms a pair of diastereomers, both of them are optically active. Also, X with C2H5ONa in C2H5OH gives a single product Y (C7H10). Y on treatment with ozone followed by reduction with (CH3)2S gives 1, 3,-cyclopentanedione as one product. Q. The correct statement concerning product(s) formed when Y is treated with excess of HCI is- a)A pair of enantiomers is formed in equal amount
- b)Two pairs of diastereomers are forme
- c)Only a meso-dichloride is formed
- d)Only one pair of diastereomers is formed
Correct answer is option 'B'. Can you explain this answer?
Passage II
An organic compound X (C7H11Br) shows optical isomerism as well as decolourises brown colour of bromine water solution. X on treatment with HBr in the absence of a peroxide forms a pair of diastereomers, both of them are optically active. Also, X with C2H5ONa in C2H5OH gives a single product Y (C7H10). Y on treatment with ozone followed by reduction with (CH3)2S gives 1, 3,-cyclopentanedione as one product.
Q. The correct statement concerning product(s) formed when Y is treated with excess of HCI is
a)
A pair of enantiomers is formed in equal amount
b)
Two pairs of diastereomers are forme
c)
Only a meso-dichloride is formed
d)
Only one pair of diastereomers is formed

|
Sravya Banerjee answered |
Diastereomers are always chiral, and always different from one another. Diastereomers occur in
pairs, and each has
two chiral centers. In the original classic diastereomer, the chirality of one of them would be ( for example ) “ R, S “, and the other would be “ R, R
Passage IAn unknown compound X (C5HgBr) does not decolourise purple colour of alkaline permanganate solution. Upon treatment with C2H5OK/C2H5OH, X gives Y (C5H8) as only structural isomer. Catalytic hydrogenation of Y gives methyl cyclobutane. Ozonolysis of Y gives Z (C5H8O2) which can exist as a pair of enantiomers.Q.
What is true regarding reaction of Y with cold, dilute alkaline KMnO4 solution?- a)A pure enantiomer of Y gives racemic mixture
- b)A pure enantiomer of Y gives a pair of achiral isomers
- c)A pure enantiomer of Y gives a pair of optically active diastereomers
- d)Y is achiral.it gives racemic mixture
Correct answer is option 'C'. Can you explain this answer?
Passage I
An unknown compound X (C5HgBr) does not decolourise purple colour of alkaline permanganate solution. Upon treatment with C2H5OK/C2H5OH, X gives Y (C5H8) as only structural isomer. Catalytic hydrogenation of Y gives methyl cyclobutane. Ozonolysis of Y gives Z (C5H8O2) which can exist as a pair of enantiomers.
Q.
What is true regarding reaction of Y with cold, dilute alkaline KMnO4 solution?
What is true regarding reaction of Y with cold, dilute alkaline KMnO4 solution?
a)
A pure enantiomer of Y gives racemic mixture
b)
A pure enantiomer of Y gives a pair of achiral isomers
c)
A pure enantiomer of Y gives a pair of optically active diastereomers
d)
Y is achiral.it gives racemic mixture

|
Ameya Mukherjee answered |
A diastereomer is a stereoisomer with two or more stereocenters and the isomers are not mirror images of each other.
If a molecule has more than one stereocenter and every single stereocenter isn’t in the opposite direction then they are diastereomers. diastereomers will always have non-identical (but non-opposite) R,S designations.
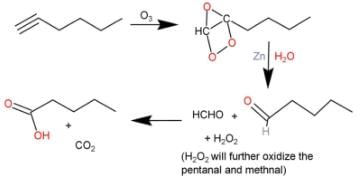
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has fo ur choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. What is/are true about ozonolysis of 1-hexyne followed by aqueous work-up?- a)Pentanoic acid and CO2 are formed
- b)Hydrolysis of ozonide of alkyne form H2O2 by product that oxidises the intermediate product into acids
- c)The ozonide has a C— C bond
- d)Pentanal and methanal are formed
Correct answer is option 'A,B,C'. Can you explain this answer?
Direction (Q. Nos. 9 - 12) This section contains 4 multiple choice questions. Each question has fo ur choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. What is/are true about ozonolysis of 1-hexyne followed by aqueous work-up?
a)
Pentanoic acid and CO2 are formed
b)
Hydrolysis of ozonide of alkyne form H2O2 by product that oxidises the intermediate product into acids
c)
The ozonide has a C— C bond
d)
Pentanal and methanal are formed
|
|
Pooja Shah answered |
The correct answers are Options A, B and C.

A hydrocarbon X (C14H22)o n treatm ent with H2/Pt gives C14H26. Also X on treatm ent with alkaline KMnO4 followed by hydrolysis of products yields C7H12O2 which on further heating with soda lime gives cyclohexane. Hence, X is- a)
- b)
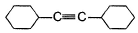
- c)
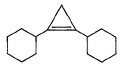
- d)
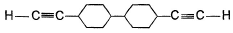
Correct answer is option 'B'. Can you explain this answer?
A hydrocarbon X (C14H22)o n treatm ent with H2/Pt gives C14H26. Also X on treatm ent with alkaline KMnO4 followed by hydrolysis of products yields C7H12O2 which on further heating with soda lime gives cyclohexane. Hence, X is
a)
b)
c)
d)
|
|
Neha Joshi answered |
The correct answers are option B.
As only 4 hydrogen atoms are increased after hydrogenation, there should be only 1 3× bond. So option B is correct.
As only 4 hydrogen atoms are increased after hydrogenation, there should be only 1 3× bond. So option B is correct.
Photochemical chlorination of alkane is initiated by process called:- a)Homolysis
- b)Pyrolysis
- c)Substitution
- d)Peroxidation
Correct answer is option 'A'. Can you explain this answer?
Photochemical chlorination of alkane is initiated by process called:
a)
Homolysis
b)
Pyrolysis
c)
Substitution
d)
Peroxidation
|
|
Rahul Bansal answered |
Photochemical chlorination of alkane take place by free radical mechanism which are possible by Homolysis of C - C bond
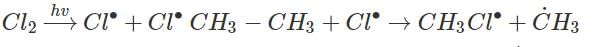
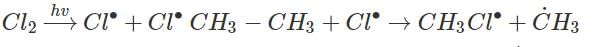
What is the major formed in the reaction?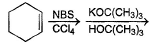
- a)

- b)

- c)

- d)

Correct answer is option 'C'. Can you explain this answer?
What is the major formed in the reaction?
a)
b)
c)
d)
|
|
Pranavi Chopra answered |
For option a
12 = [Ne]3s2 , 20 = [Ar]4s2 , 4 = [He]2s2 , 88 = [Ra]7s2.
For option b
8 = [He]2s22p4 , 16 = [Ne]3s23p4 , 34 = [Ar]4s23d104p4 , 2 = 1s2
We can see that only in option a, the last electrons enter in similar group. So, option a is correct.
Similarly we can check for option c and d as done above. However they won't fall in the same group.
12 = [Ne]3s2 , 20 = [Ar]4s2 , 4 = [He]2s2 , 88 = [Ra]7s2.
For option b
8 = [He]2s22p4 , 16 = [Ne]3s23p4 , 34 = [Ar]4s23d104p4 , 2 = 1s2
We can see that only in option a, the last electrons enter in similar group. So, option a is correct.
Similarly we can check for option c and d as done above. However they won't fall in the same group.
Which starting material should be used to produce the compound shown below?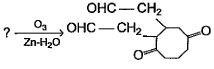
- a)
- b)
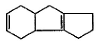
- c)
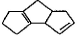
- d)

Correct answer is option 'B'. Can you explain this answer?
Which starting material should be used to produce the compound shown below?
a)
b)
c)
d)
|
|
Suresh Iyer answered |
The correct answer is option B

Which of the following reagent(s) can be used to distinguish between 1-hexyne and 2-hexyne?- a)Ammoniacal AgNO3 solution
- b)Ammoniacal solution of Cu(11) tartarate
- c)Cold, dilute and alkaline KMnO4
- d)H2SO4/HgSO4
Correct answer is option 'A,B'. Can you explain this answer?
Which of the following reagent(s) can be used to distinguish between 1-hexyne and 2-hexyne?
a)
Ammoniacal AgNO3 solution
b)
Ammoniacal solution of Cu(11) tartarate
c)
Cold, dilute and alkaline KMnO4
d)
H2SO4/HgSO4
|
|
Raghav Bansal answered |
The correct answers are Options A and B.
AgNO3 is called Tollen's reagent.
It reacts with terminal alkynes (1-hexyne) but not with alkenes (1-hexene).
2CH≡CCH2CH2CH3 + 2AgNO3 + 2NH4OH——›
1-hexyne
2CH≡CCH2CH2CH3Ag + 2NH4NO3 + 2H2O
AgNO3 is called Tollen's reagent.
It reacts with terminal alkynes (1-hexyne) but not with alkenes (1-hexene).
2CH≡CCH2CH2CH3 + 2AgNO3 + 2NH4OH——›
1-hexyne
2CH≡CCH2CH2CH3Ag + 2NH4NO3 + 2H2O
Terminal alkynes (hex-1-yne) react with ammoniacal
Cu2Cl2 solution. Tollens reagent, etc.
Cu2Cl2 solution. Tollens reagent, etc.
Chapter doubts & questions for Hydrocarbons and Nomenclature - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Hydrocarbons and Nomenclature - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup