All Exams >
Year 4 >
Science for Year 4 >
All Questions
All questions of Properties of Materials for Year 4 Exam
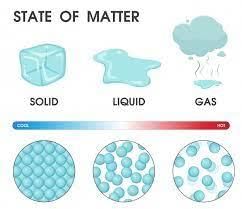
Which matter is normally hard?- a)Liquid
- b)Solid
- c)Gases
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Which matter is normally hard?
a)
Liquid
b)
Solid
c)
Gases
d)
All of these
|
|
Sudhir Mehta answered |
In the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. As a result, solids have a definite shape and volume. Most solids are hard.
State whether the following statement is True or False:The substance that dissolves in a liquid to form a solution is called a solute.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
State whether the following statement is True or False:
The substance that dissolves in a liquid to form a solution is called a solute.
a)
True
b)
False
|
|
Riya Singh answered |
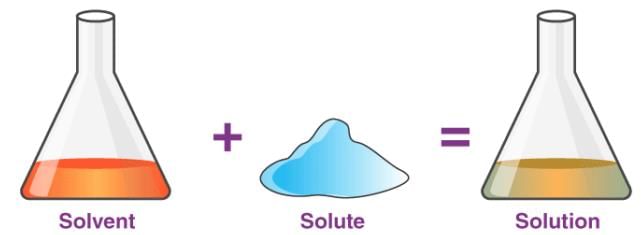
When a substance dissolves in a liquid to make a solution, it is called a solute. For example, when you mix salt in water and it disappears, salt is the solute. This makes the statement TRUE.
Which process would be used to separate sugar from a sugar-water solution?- a)Filtration
- b)Sedimentation
- c)Evaporation
- d)Decantation
Correct answer is option 'C'. Can you explain this answer?
a)
Filtration
b)
Sedimentation
c)
Evaporation
d)
Decantation
|
|
Subset Academy answered |
Evaporation is used to separate sugar from a sugar-water solution. By heating the solution, the water evaporates, leaving behind the sugar crystals. This method works because sugar dissolves in water but does not evaporate with it.
What is the main reason why gases are highly compressible compared to liquids and solids?- a)High density
- b)Low intermolecular space
- c)High kinetic energy
- d)Large intermolecular space
Correct answer is option 'D'. Can you explain this answer?
a)
High density
b)
Low intermolecular space
c)
High kinetic energy
d)
Large intermolecular space
|
|
Freak Artworks answered |
Gases are highly compressible because they have large intermolecular spaces between particles, allowing them to be compressed into a smaller volume. This is due to the negligible forces of attraction between gas particles.
Which state of matter is characterized by the highest rate of diffusion?- a)Solid
- b)Liquid
- c)Gas
- d)Plasma
Correct answer is option 'C'. Can you explain this answer?
a)
Solid
b)
Liquid
c)
Gas
d)
Plasma
|
|
Subset Academy answered |
Gases exhibit the highest rate of diffusion because their particles are widely spaced and move freely, allowing them to mix rapidly with other gases. This high mobility contrasts with the slow diffusion in solids and liquids.
When a gas changes into a liquid, it is called _______.- a)evaporation
- b)condensation
- c)solidification
- d)melting
Correct answer is option 'B'. Can you explain this answer?
When a gas changes into a liquid, it is called _______.
a)
evaporation
b)
condensation
c)
solidification
d)
melting
|
|
Sudhir Mehta answered |
Conversion of gases into liquids is called condensation.
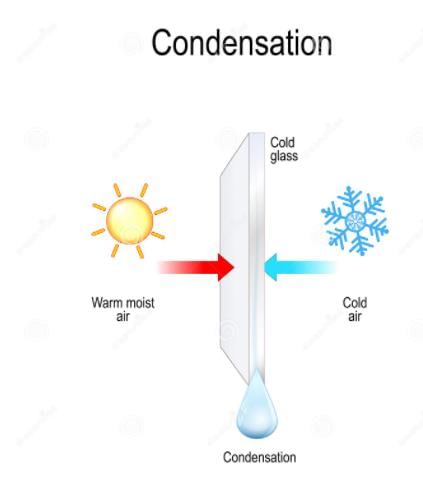
What is the role of a solute in a solution?- a)To dissolve the solvent
- b)To form a mixture with the solvent
- c)To evaporate from the solution
- d)To separate from the solvent
Correct answer is option 'B'. Can you explain this answer?
a)
To dissolve the solvent
b)
To form a mixture with the solvent
c)
To evaporate from the solution
d)
To separate from the solvent
|
|
Tanishq Dasgupta answered |
The Role of a Solute in a Solution
In a solution, a solute plays a crucial role in creating a homogenous mixture with the solvent. Let's explore this concept further.
Definition of a Solute
- A solute is a substance that is dissolved in a solvent.
- Common examples include salt in water or sugar in tea.
Formation of a Mixture
- When a solute is added to a solvent, it disperses evenly throughout the solvent.
- This process creates a solution, where the solute is no longer visible as a separate entity.
Interaction with the Solvent
- The solute interacts with the solvent molecules, breaking into smaller particles.
- This interaction is vital for the solute to dissolve effectively.
Importance of Homogeneity
- A solution is characterized by its uniform composition, meaning every part of the solution has the same properties.
- The solute ensures that the solution is consistent, making it useful for various applications, such as cooking or chemical reactions.
Conclusion
- In summary, the solute's main role is to mix with the solvent to form a solution.
- This mixture allows for the properties of both substances to be utilized in various practical scenarios.
By understanding the role of a solute, we gain insight into the nature of solutions and their importance in everyday life.
In a solution, a solute plays a crucial role in creating a homogenous mixture with the solvent. Let's explore this concept further.
Definition of a Solute
- A solute is a substance that is dissolved in a solvent.
- Common examples include salt in water or sugar in tea.
Formation of a Mixture
- When a solute is added to a solvent, it disperses evenly throughout the solvent.
- This process creates a solution, where the solute is no longer visible as a separate entity.
Interaction with the Solvent
- The solute interacts with the solvent molecules, breaking into smaller particles.
- This interaction is vital for the solute to dissolve effectively.
Importance of Homogeneity
- A solution is characterized by its uniform composition, meaning every part of the solution has the same properties.
- The solute ensures that the solution is consistent, making it useful for various applications, such as cooking or chemical reactions.
Conclusion
- In summary, the solute's main role is to mix with the solvent to form a solution.
- This mixture allows for the properties of both substances to be utilized in various practical scenarios.
By understanding the role of a solute, we gain insight into the nature of solutions and their importance in everyday life.
State whether the following statement is True or False:Some solids that dissolve in water are called soluble substances.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
State whether the following statement is True or False:
Some solids that dissolve in water are called soluble substances.
a)
True
b)
False
|
|
Edgy Education answered |
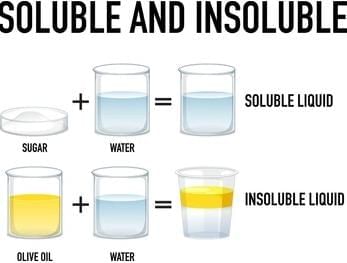
- When a solid substance dissolves in water, we call it a soluble substance. For example, when you add sugar to water and stir it, the sugar disappears because it dissolves in the water, forming a sugar water solution. So, the statement is True.
- Salt, sugar, soap, and orange juice are some examples of substances that can dissolve in water.
What method is used to separate a mixture of sand and water?- a)Filtration
- b)Evaporation
- c)Sedimentation and Decantation
- d)Condensation
Correct answer is option 'C'. Can you explain this answer?
a)
Filtration
b)
Evaporation
c)
Sedimentation and Decantation
d)
Condensation

|
Nclex Coaching Centre answered |
Sedimentation and decantation are used to separate sand from water. Sand settles at the bottom of the glass, and the clear water can be poured off, leaving the sand behind. This method is effective for separating insoluble solids from liquids.
What is the outcome when a substance undergoes condensation?- a)It changes from a liquid to a gas
- b)It changes from a solid to a liquid
- c)It changes from a gas to a liquid
- d)It changes from a gas to a solid
Correct answer is option 'C'. Can you explain this answer?
a)
It changes from a liquid to a gas
b)
It changes from a solid to a liquid
c)
It changes from a gas to a liquid
d)
It changes from a gas to a solid
|
|
Riya Singh answered |
Condensation involves a substance changing from a gas to a liquid. For example, water vapor in the air condenses into liquid water when cooled, such as when steam forms droplets on a cold surface.
Choose the correct option from the following on the basis of the packing of molecules.- a)Solid > Liquid > Gas
- b)Gas > Solid > Liquid
- c)Liquid > Gas > Solid
- d)Solid > Gas > Liquid
Correct answer is option 'A'. Can you explain this answer?
Choose the correct option from the following on the basis of the packing of molecules.
a)
Solid > Liquid > Gas
b)
Gas > Solid > Liquid
c)
Liquid > Gas > Solid
d)
Solid > Gas > Liquid
|
|
Sudhir Mehta answered |
Solid > Liquid > Gas. Solids have the most tightly packed molecules and gases have the least packed molecules.
Which of the following is an example of an insoluble substance in water?- a)Sugar
- b)Salt
- c)Tea leaves
- d)Coffee
Correct answer is option 'C'. Can you explain this answer?
a)
Sugar
b)
Salt
c)
Tea leaves
d)
Coffee
|
|
Edgy Education answered |
Tea leaves are an example of an insoluble substance in water. Unlike sugar and salt, which dissolve, tea leaves do not dissolve in water. They remain separate and can be filtered out from the liquid.
What changes occur to the molecules of a substance during evaporation?- a)They move closer together
- b)They move further apart
- c)They become more rigid
- d)They lose their energy
Correct answer is option 'B'. Can you explain this answer?
a)
They move closer together
b)
They move further apart
c)
They become more rigid
d)
They lose their energy

|
Nclex Coaching Centre answered |
During evaporation, the molecules of a substance move further apart as they gain enough energy to escape the liquid state and enter the gas phase. This process involves an increase in kinetic energy and movement of molecules.
What is the state of matter that takes the shape of its container?
- a)Plasma
- b)Solid
- c)Liquid
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
What is the state of matter that takes the shape of its container?
a)
Plasma
b)
Solid
c)
Liquid
d)
None of these

|
Nclex Coaching Centre answered |
Liquids are cool because they can change their shape to fit into any container they are poured into. Imagine pouring juice from a glass to a bowl - the juice takes the shape of the bowl. That's what liquids do!
What happens to the rate of diffusion when a substance changes from a solid to a liquid?- a)It decreases
- b)It stays the same
- c)It increases
- d)It stops
Correct answer is option 'C'. Can you explain this answer?
a)
It decreases
b)
It stays the same
c)
It increases
d)
It stops
|
|
Riya Singh answered |
The rate of diffusion increases when a substance changes from a solid to a liquid. In liquids, particles are less tightly packed and can move more freely compared to solids, allowing for faster diffusion.
Chapter doubts & questions for Properties of Materials - Science for Year 4 2025 is part of Year 4 exam preparation. The chapters have been prepared according to the Year 4 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Year 4 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Properties of Materials - Science for Year 4 in English & Hindi are available as part of Year 4 exam.
Download more important topics, notes, lectures and mock test series for Year 4 Exam by signing up for free.
Science for Year 4
22 videos|34 docs|11 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









