All Exams >
MCAT >
General Chemistry for MCAT >
All Questions
All questions of Dot Structures for MCAT Exam
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?- a)trigonal pyramidal
- b)square pyramidal
- c)square planar
- d)tetrahedral
Correct answer is option 'C'. Can you explain this answer?
Cis-platin [PtCl2(NH3)2] is an anti-cancer drug that reacts by binding to and causing the crosslinking of DNA ultimately leading to apoptosis and is the geometric isomer of trans-platin. Given that cis-platin exhibits stereochemistry, what would you predict is the molecular geometry of the anticancer drug?
a)
trigonal pyramidal
b)
square pyramidal
c)
square planar
d)
tetrahedral
|
|
Isaac Reed answered |
Explanation:
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
In order to determine the molecular geometry of cis-platin [PtCl2(NH3)2], we need to consider the arrangement of atoms around the central metal atom (platinum, Pt) and the presence of any lone pairs of electrons.
1. Central Atom:
The central atom in cis-platin is platinum (Pt).
2. Ligands:
The ligands in cis-platin are two chloride ions (Cl-) and two ammonia molecules (NH3).
3. Coordination Number:
The coordination number of platinum is determined by the number of ligands surrounding it. In this case, there are four ligands (two chloride ions and two ammonia molecules), so the coordination number is 4.
4. Lone Pairs:
In cis-platin, the central platinum atom does not have any lone pairs of electrons.
5. Geometry:
Based on the coordination number and absence of lone pairs, the molecular geometry of cis-platin can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory).
Since there are four ligands (two chloride ions and two ammonia molecules) and no lone pairs, the electronic geometry of cis-platin is tetrahedral.
However, the question asks for the molecular geometry, which takes into account the presence of multiple ligands. In cis-platin, the two chloride ions and the two ammonia molecules are not symmetrical around the central platinum atom.
This lack of symmetry results in the cis arrangement, where the chloride ions and ammonia molecules are adjacent to each other rather than across from each other. This cis arrangement gives cis-platin its geometric isomerism.
The cis arrangement leads to the molecular geometry of square planar. In a square planar geometry, the ligands are arranged in a square plane around the central atom, with bond angles of 90 degrees.
Therefore, the correct answer is option C: square planar.
Nitrates are used to treat angina pectoris and acts to dilate the blood vessels to lower blood pressure. They exert their effects by their conversion into nitric oxide, a powerful vasodilator. What is the bond order and fractional charge on each oxygen of the nitrate ion?- a)Bond order of 1(1/3) fractional charge of −1/3
- b)Bond order of 2 fractional charge of −1/3
- c)Bond order of 1(1/3) fractional charge of −2/3
- d)Bond order of 2 fractional charge of −2/3
Correct answer is option 'C'. Can you explain this answer?
Nitrates are used to treat angina pectoris and acts to dilate the blood vessels to lower blood pressure. They exert their effects by their conversion into nitric oxide, a powerful vasodilator. What is the bond order and fractional charge on each oxygen of the nitrate ion?
a)
Bond order of 1(1/3) fractional charge of −1/3
b)
Bond order of 2 fractional charge of −1/3
c)
Bond order of 1(1/3) fractional charge of −2/3
d)
Bond order of 2 fractional charge of −2/3
|
|
Ayesha Joshi answered |
We have to determine that the resonance structure of nitrate, which is NO3⁻. Nitrogen seems to be the central atom since it can form 4 bonds, while oxygen cannot. We start with each oxygen single bonded.
When oxygen is single bonded, it has a formal charge of -1, which means in total -3 with the three oxygens. This is not a feasible Lewis dot structure, so let’s move on by making one of the bonds a double bond:
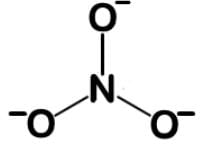

When oxygen is double bonded, it is uncharged. When nitrogen has four bonds, it takes on a charge of +1. When oxygen is single bonded, it takes on a charge of -1, and we have 2 for -2. In total, we have +1 and -2, giving us the -1 for the overall charge we are looking for:
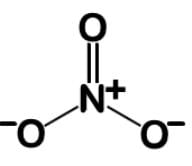

The bond order is the total number of bonds divided by the number of attaching atoms, which gives 4/3 or 1.33
The fractional charge is the total charge on the attaching atoms divided by the number of attaching ions, which is -⅔.
The correct answer is a bond order of 1⅓ and a fractional charge of -⅔ .
Determining Lewis dot structures can be used to determine whether a compound has any bond dipoles and consequently any molecular dipole. Which of the following compounds does NOT have a molecular dipole?- a)NH3
- b)PO33-
- c)CIO3-
- d)SO3
Correct answer is option 'D'. Can you explain this answer?
Determining Lewis dot structures can be used to determine whether a compound has any bond dipoles and consequently any molecular dipole. Which of the following compounds does NOT have a molecular dipole?
a)
NH3
b)
PO33-
c)
CIO3-
d)
SO3
|
|
Ayesha Joshi answered |
The hypochlorite ion ClO3- has 2 double-bonded oxygens and 1 single-bonded oxygen to the chlorine central atom. Since chlorine can have 7 bonds, with 5 bonds to oxygen, there are 2 electrons remaining as an unbonded pair. Hypochlorite has the trigonal pyramidal molecular geometry.
The phosphite ion PO33- has a central phosphorus atom with 3 single-bonded oxygens each with a negative charge. Since phosphorus has 5 valence electrons, there are 2 electrons remaining for a lone pair. Phosphite has the trigonal pyramidal molecular geometry.
Ammonia NH3 , has a central nitrogen with 3 single-bonded hydrogens. Since nitrogen has 5 valence electrons, there are 2 electrons remaining for an unbonded pair.
Sulfur trioxide SO3 has a central sulfur atom attached to 3 oxygens with double bonds. The molecular geometry is trigonal planar, and it has no molecular dipole.
Carbon monoxide (CO) is a potent competitive inhibitor of hemoglobin, and it has an affinity for hemoglobin over 200 times greater than oxygen. What is the formal charge of the oxygen on the molecule?- a)+1
- b)0
- c)-1
- d)-2
Correct answer is option 'A'. Can you explain this answer?
Carbon monoxide (CO) is a potent competitive inhibitor of hemoglobin, and it has an affinity for hemoglobin over 200 times greater than oxygen. What is the formal charge of the oxygen on the molecule?
a)
+1
b)
0
c)
-1
d)
-2
|
|
Ayesha Joshi answered |
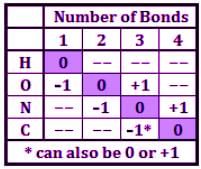
The HONC (pronounced hunk or honk) shortcut, which correspond to 1, 2, 3, or 4 bonds, will be employed for all questions in this set. When these four atoms have the corresponding bonds, then the atoms are neutral. One less bond to that atom makes its more negative by 1, and one more bond more positive by 1.
Here is a chart that summarizes the shortcut:
Here is a chart that summarizes the shortcut:
Start by drawing a skeleton of C−O and consider the double bond. Looking at the formal charges, carbon would have no formal charge, as well as the oxygen. This would seem correct, but remember the octet rule. It is not much of a rule since C, N, O, and F are the only four elements that must adhere to the rule. Unless there is a carbene (:CH2), carbon usually needs to fill its octet even though C=O seems to be a feasible Lewis dot structure.
n analyzing the triple bond C≡O, carbon has a formal charge of -1, and oxygen a formal charge of +1. Both have their octets filled, and so here is the Lewis dot structure:
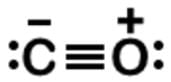
To find the formal charge of the oxygen, start with the number of valence electrons, which is 6. Subtract any unbonded electrons, which is 2, and then subtract one-half of any bonded electrons, which is 3.
Generally in determining structures, the more electronegative atom tends to have a negative formal charge, and the more electropositive a positive one. Despite being more electronegative, oxygen does not get assigned the automatic negative charge but has instead a formal charge of +1.
Which of the following statements most accurately describes the difference between assigning formal charge and oxidation state?- a)In determining formal charge, treat the bonds as if they are ionic bonds, and generally do not assign a charge greater than 1.
- b)The atoms can be assigned any value from -4 to +7 in determining oxidation states.
- c)In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons.
- d)The assigned formal charges and oxidation states can separately add up to different values.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements most accurately describes the difference between assigning formal charge and oxidation state?
a)
In determining formal charge, treat the bonds as if they are ionic bonds, and generally do not assign a charge greater than 1.
b)
The atoms can be assigned any value from -4 to +7 in determining oxidation states.
c)
In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons.
d)
The assigned formal charges and oxidation states can separately add up to different values.
|
|
Olivia Johnson answered |
Understanding Formal Charge vs. Oxidation State
When differentiating between formal charge and oxidation state, it's essential to grasp how each is calculated and what they represent.
Formal Charge
- Formal charge is a bookkeeping tool used to help predict the stability of a molecule.
- It is calculated using the formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (Bonding Electrons/2).
- In this method, bonds are treated as if they are covalent, meaning that electrons are shared equally between atoms.
Oxidation State
- Oxidation state indicates the degree of oxidation (loss of electrons) of an atom in a compound.
- It is calculated by assigning electrons based on electronegativity; the more electronegative atom in a bond is assigned both electrons.
- This approach treats bonds as if they are ionic, reflecting the charge distribution more accurately.
Why Option C is Correct
- Option C states, "In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons."
- This is correct because oxidation states reflect the theoretical charge an atom would have if all bonds were ionic.
- It highlights the fundamental difference from formal charge, where bonds are considered covalent and shared.
Key Takeaways
- Formal charge helps evaluate molecular stability, while oxidation states indicate electron distribution.
- Understanding this distinction is crucial for analyzing chemical reactions and bonding scenarios effectively.
When differentiating between formal charge and oxidation state, it's essential to grasp how each is calculated and what they represent.
Formal Charge
- Formal charge is a bookkeeping tool used to help predict the stability of a molecule.
- It is calculated using the formula:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (Bonding Electrons/2).
- In this method, bonds are treated as if they are covalent, meaning that electrons are shared equally between atoms.
Oxidation State
- Oxidation state indicates the degree of oxidation (loss of electrons) of an atom in a compound.
- It is calculated by assigning electrons based on electronegativity; the more electronegative atom in a bond is assigned both electrons.
- This approach treats bonds as if they are ionic, reflecting the charge distribution more accurately.
Why Option C is Correct
- Option C states, "In assigning oxidation states, we treat the bonds as if they are ionic bonds where the more electronegative atom is awarded the electrons."
- This is correct because oxidation states reflect the theoretical charge an atom would have if all bonds were ionic.
- It highlights the fundamental difference from formal charge, where bonds are considered covalent and shared.
Key Takeaways
- Formal charge helps evaluate molecular stability, while oxidation states indicate electron distribution.
- Understanding this distinction is crucial for analyzing chemical reactions and bonding scenarios effectively.
Which of the following compounds does NOT have a bent molecular geometry?- a)H2O
- b)l3
- c)ClO2-
- d)SO2
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds does NOT have a bent molecular geometry?
a)
H2O
b)
l3
c)
ClO2-
d)
SO2
|
|
Ayesha Joshi answered |
Sulfur dioxide SO2 is a classic trap for linear molecular geometry due to its likeness to CO2, which is definitely linear. SO2 is bent due to the single lone pair:
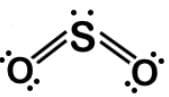
H2O is bent due to the two lone pairs on the oxygen. Here is the Lewis dot structure:
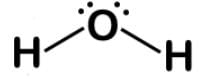
The chlorite ion ClO2- is also bent due to the two lone pairs on the chlorine. Here is the Lewis dot structure:
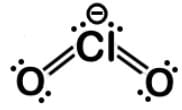
The triiodide ion I3- is not bent because it has 3 lone pairs, but it has a linear molecular geometry with a trigonal bipyramidal electronic geometry.
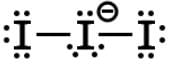
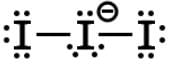
Periodic acid (H5IO6) will selectively cleave vicinal diols into two aldehyde or ketone fragments, which can be used to label the 3’-termini of RNA instead of DNA. Periodic acid can be dehydrated twice to form metal periodic acid. What are the molecular geometries of the two compounds respectively?- a)octahedral and tetrahedral
- b)trigonal bipyramidal and see-saw
- c)trigonal bipyramidal and square planar
- d)hexagonal and tetrahedral
Correct answer is option 'A'. Can you explain this answer?
Periodic acid (H5IO6) will selectively cleave vicinal diols into two aldehyde or ketone fragments, which can be used to label the 3’-termini of RNA instead of DNA. Periodic acid can be dehydrated twice to form metal periodic acid. What are the molecular geometries of the two compounds respectively?
a)
octahedral and tetrahedral
b)
trigonal bipyramidal and see-saw
c)
trigonal bipyramidal and square planar
d)
hexagonal and tetrahedral
|
|
Olivia Johnson answered |
Understanding Periodic Acid (H5IO6)
Periodic acid, H5IO6, is a chemical compound known for its ability to cleave vicinal diols into aldehyde or ketone fragments. Its molecular geometry is essential to understanding its reactivity and interactions.
Molecular Geometry of H5IO6
- The molecular geometry of periodic acid is octahedral.
- This is due to the presence of six substituents around the central iodine atom, which follows the VSEPR theory.
Metal Periodic Acid
- When periodic acid undergoes dehydration, it can form metal periodic acid.
- The geometry of metal periodic acid is typically tetrahedral.
- This results from the coordination of metal ions with the periodic acid structure, leading to a four-coordinate environment.
Summary of Molecular Geometries
- H5IO6: Octahedral
- Metal Periodic Acid: Tetrahedral
Conclusion
Thus, the correct answer to the question regarding the molecular geometries of periodic acid and metal periodic acid is option 'A': octahedral and tetrahedral. This distinction is crucial for applications involving these compounds, especially in biochemical contexts like RNA labeling.
Periodic acid, H5IO6, is a chemical compound known for its ability to cleave vicinal diols into aldehyde or ketone fragments. Its molecular geometry is essential to understanding its reactivity and interactions.
Molecular Geometry of H5IO6
- The molecular geometry of periodic acid is octahedral.
- This is due to the presence of six substituents around the central iodine atom, which follows the VSEPR theory.
Metal Periodic Acid
- When periodic acid undergoes dehydration, it can form metal periodic acid.
- The geometry of metal periodic acid is typically tetrahedral.
- This results from the coordination of metal ions with the periodic acid structure, leading to a four-coordinate environment.
Summary of Molecular Geometries
- H5IO6: Octahedral
- Metal Periodic Acid: Tetrahedral
Conclusion
Thus, the correct answer to the question regarding the molecular geometries of periodic acid and metal periodic acid is option 'A': octahedral and tetrahedral. This distinction is crucial for applications involving these compounds, especially in biochemical contexts like RNA labeling.
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3 or ClF5. What is the molecular geometry of chlorine trifluoride?- a)trigonal planar
- b)trigonal pyramidal
- c)T-shaped
- d)trigonal bipyramidal
Correct answer is option 'C'. Can you explain this answer?
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3 or ClF5. What is the molecular geometry of chlorine trifluoride?
a)
trigonal planar
b)
trigonal pyramidal
c)
T-shaped
d)
trigonal bipyramidal
|
|
Olivia Johnson answered |
Molecular Geometry of Chlorine Trifluoride
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Xanthocillin is a natural product extracted from the mold Penicillium notatum that has been used as an antibiotic and also contains the functional group isonitrile RNC. Which of the following resonance structures can be found for the functional group?- a)
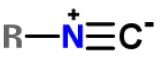
- b)
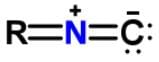
- c)
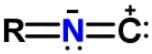
- d)
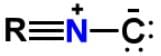
Correct answer is option 'A'. Can you explain this answer?
Xanthocillin is a natural product extracted from the mold Penicillium notatum that has been used as an antibiotic and also contains the functional group isonitrile RNC. Which of the following resonance structures can be found for the functional group?
a)
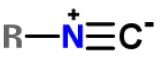
b)
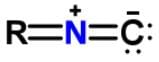
c)
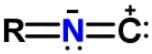
d)
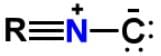
|
|
Ayesha Joshi answered |
Applying the HONC strategy, when nitrogen has 4 bonds, it takes on a charge of +1.
To have a complete octet, carbon must have at least 3 bonds and when it does it takes on a charge of -1.
R is representative of any alkyl group, and R can be single, double, or triple bonded depending on whether it is -CH, -CH2 or -CH3.
The correct resonance structure is:
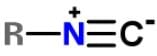
Thiocyanate is a potent competitive inhibitor of the thyroid sodium-iodide symporter and should be avoided by individuals suffering from hypothyroidism. What is the formal charge of the sulfur in the thiocyanate ion SCN−?I. -2
II. -1
III. 0
IV. +1- a)I and II
- b)II and III
- c)II and IV
- d)III only
Correct answer is option 'B'. Can you explain this answer?
Thiocyanate is a potent competitive inhibitor of the thyroid sodium-iodide symporter and should be avoided by individuals suffering from hypothyroidism. What is the formal charge of the sulfur in the thiocyanate ion SCN−?
I. -2
II. -1
III. 0
IV. +1
II. -1
III. 0
IV. +1
a)
I and II
b)
II and III
c)
II and IV
d)
III only
|
|
Ayesha Joshi answered |
Treat the sulfur as if it were an oxygen since they are both in the same group, remembering of course that sulfur can expand its octet.
Start with the skeleton S−C−N and quickly give carbon four bonds to complete its octet. There are only two options: S=C=N and S−C≡N. S≡C−N can be quickly eliminated since N cannot have only 1 bond.
Looking at S=C=N, when sulfur like oxygen has 2 bonds and carbon has 4 bonds, they both have no formal charge. When nitrogen has 2 bonds, it has a formal charge of -1.
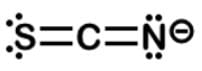
Looking at S-C≡N, when sulfur like oxygen has 1 bond, it has a formal charge of -1. When nitrogen has 3 bonds and carbon has 4 bonds, they both have no formal charge.
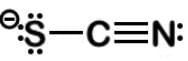
From the Lewis dot structures above, sulfur can have a formal charge of -1 or 0.
Chapter doubts & questions for Dot Structures - General Chemistry for MCAT 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Dot Structures - General Chemistry for MCAT in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
General Chemistry for MCAT
164 videos|11 docs|16 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup









