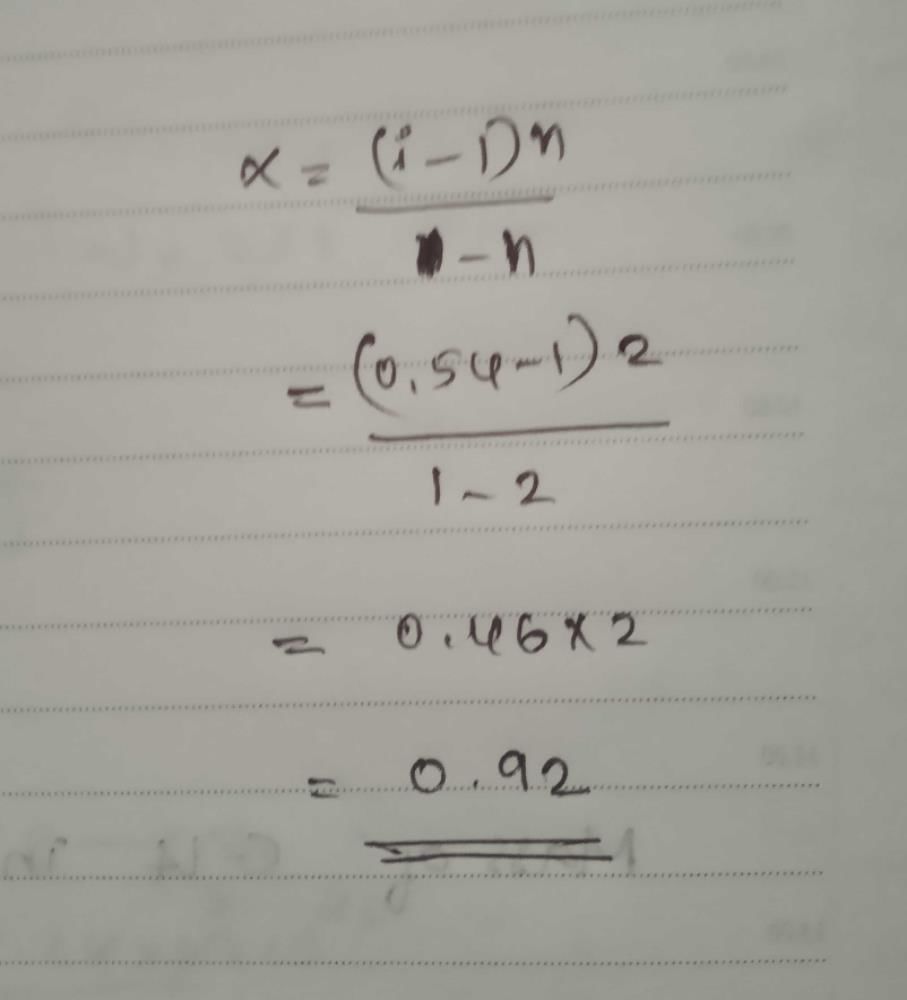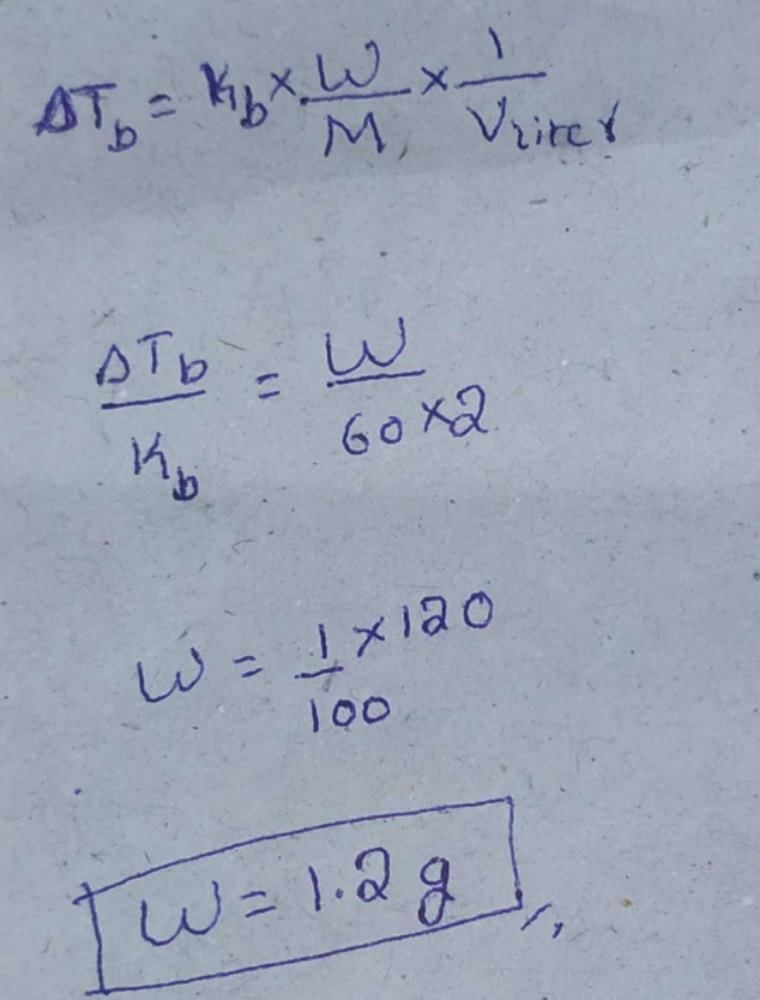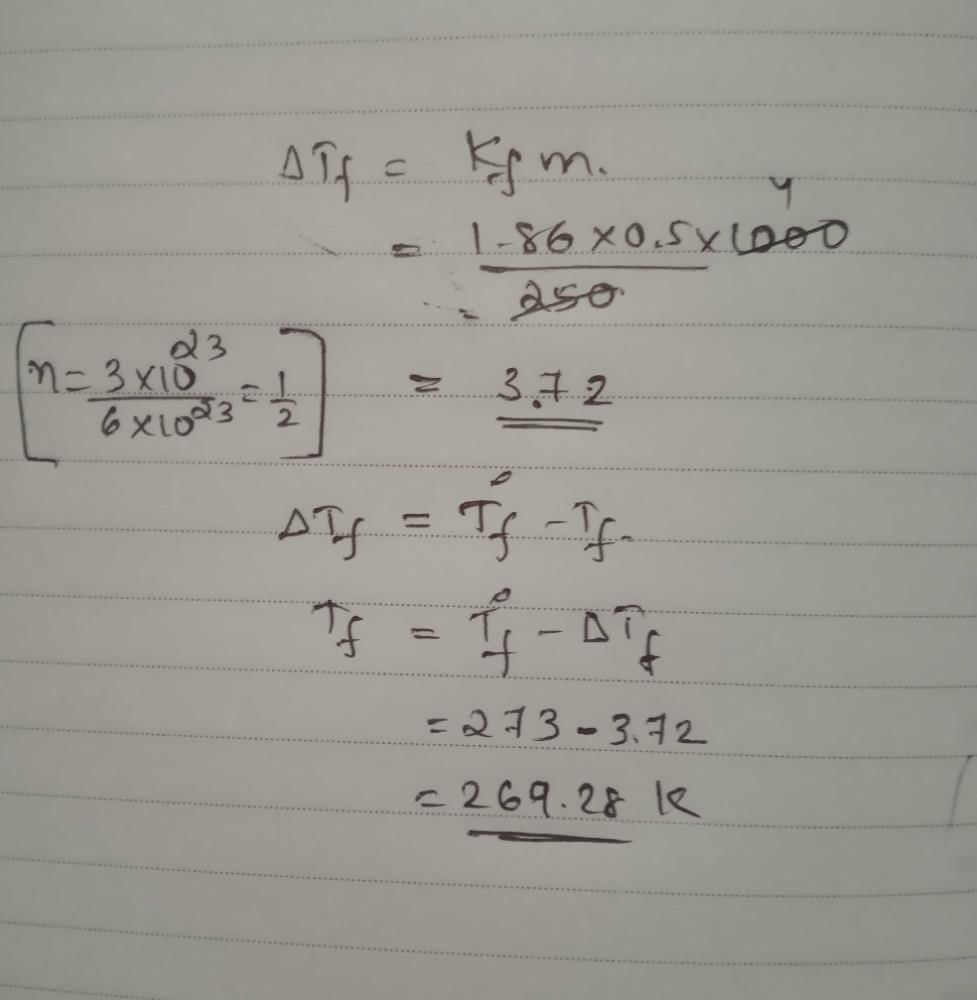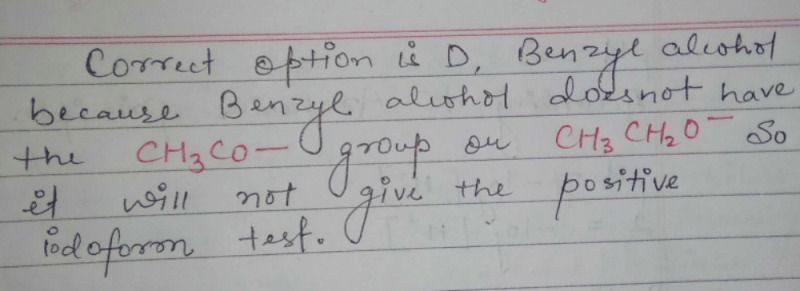All Exams >
NEET >
NEET Mock Test Series - Updated 2026 Pattern >
All Questions
All questions of Chemistry: Topic-wise Test for NEET Exam
Chlorobenzene on fusing with solid NaOH gives- a)Benzene
- b)Phenol
- c)Benzoic acid
- d)Benzene chloride
Correct answer is option 'B'. Can you explain this answer?
a)
Benzene
b)
Phenol
c)
Benzoic acid
d)
Benzene chloride
|
|
Anjali Chawla answered |
OH replaces chlorine ion and forms Phenol as a product. That's why option b is correct.
Methyl bromide and Ethyl bromide is mixed with equal proportions and the mixture is treated with metallic sodium. Which of the following is not expected to form- a)Ethane
- b)Propane
- c) Butane
- d) Pentane
Correct answer is option 'D'. Can you explain this answer?
a)
Ethane
b)
Propane
c)
Butane
d)
Pentane
|
|
Mirza Asad answered |
The reaction is wurtz when symmetrical alkyl halide react with sodium in presence of dryether form only one product.but unsymmetrical alkyl halide react so different product formation occur.
Alcohol can be obtained by all methods except- a)
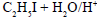
- b)
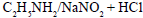
- c)
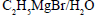
- d)
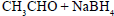
Correct answer is option 'C'. Can you explain this answer?
a)
b)
c)
d)
|
|
Raghav Bansal answered |
Answer :C
Solution :
C2H5MgBr + H2O = Mg(OH)Br + C2H6(Ethane)
Increasing order of acid strength among tertiarybutanol, isopropanol and ehanol is- a)Ethanol, isoporpanol, tertiary butanol
- b)Tertiary butanol, isopropanol, ethanol
- c)Isopropanol, tertiary butanol, ehtanol
- d)none of these
Correct answer is option 'B'. Can you explain this answer?
a)
Ethanol, isoporpanol, tertiary butanol
b)
Tertiary butanol, isopropanol, ethanol
c)
Isopropanol, tertiary butanol, ehtanol
d)
none of these
|
|
Kathiroli Krishnamurth answered |
Increase in R increases the e+ve of the element ,soacidic strength decreases as R group increase in the compound. so acidic strength is in the order of ETHANOL > isopropanol > tertiarybutanol...so b is crt
Aryl halides are less reactive towards neucliophilic substitution reactions as compared to alkyl halides due to- a)Resonance stabilisation
- b) Larger carbon–halogen bond
- c)Formation of less stable carbonium ion
- d)Inductive effect
Correct answer is option 'A'. Can you explain this answer?
a)
Resonance stabilisation
b)
Larger carbon–halogen bond
c)
Formation of less stable carbonium ion
d)
Inductive effect
|
|
Upasana Sharma answered |
**Resonance Stabilization of Aryl Halides**
**Introduction**
Aryl halides (also known as aryl halogen compounds) are organic compounds that contain a halogen atom (such as chlorine, bromine, or iodine) attached to an aromatic ring. In comparison to alkyl halides, aryl halides are less reactive towards nucleophilic substitution reactions. The main reason behind this difference in reactivity is the resonance stabilization of aryl halides.
**Resonance Stabilization**
Resonance stabilization refers to the delocalization of electrons in a molecule or ion through the overlap of p-orbitals. In the case of aryl halides, the aromatic ring (such as benzene) possesses a system of conjugated π electrons. These π electrons are delocalized over the entire ring, leading to resonance stabilization.
**Resonance Structures**
In aryl halides, the resonance structures can be represented as follows:

**Resonance Stabilization Effect**
The resonance stabilization of aryl halides makes it difficult for a nucleophile to attack the electrophilic carbon atom attached to the halogen. This is because the electrons of the aromatic ring are already delocalized and contribute to the stability of the molecule. As a result, the electron density on the carbon atom is reduced, making it less susceptible to nucleophilic attack.
**Comparison with Alkyl Halides**
In contrast, alkyl halides do not possess a conjugated system of π electrons and, therefore, lack resonance stabilization. The electron density on the carbon atom in alkyl halides is higher, making them more prone to nucleophilic attack.
**Other Factors**
While resonance stabilization is the primary reason for the decreased reactivity of aryl halides towards nucleophilic substitution reactions, other factors such as the larger carbon-halogen bond length and the formation of less stable carbonium ions also contribute to their lower reactivity. However, these factors are not as significant as the resonance stabilization effect.
**Conclusion**
In conclusion, aryl halides are less reactive towards nucleophilic substitution reactions compared to alkyl halides due to their resonance stabilization effect. The delocalization of electrons in the conjugated π system of the aromatic ring reduces the electron density on the carbon atom, making it less susceptible to nucleophilic attack.
**Introduction**
Aryl halides (also known as aryl halogen compounds) are organic compounds that contain a halogen atom (such as chlorine, bromine, or iodine) attached to an aromatic ring. In comparison to alkyl halides, aryl halides are less reactive towards nucleophilic substitution reactions. The main reason behind this difference in reactivity is the resonance stabilization of aryl halides.
**Resonance Stabilization**
Resonance stabilization refers to the delocalization of electrons in a molecule or ion through the overlap of p-orbitals. In the case of aryl halides, the aromatic ring (such as benzene) possesses a system of conjugated π electrons. These π electrons are delocalized over the entire ring, leading to resonance stabilization.
**Resonance Structures**
In aryl halides, the resonance structures can be represented as follows:

**Resonance Stabilization Effect**
The resonance stabilization of aryl halides makes it difficult for a nucleophile to attack the electrophilic carbon atom attached to the halogen. This is because the electrons of the aromatic ring are already delocalized and contribute to the stability of the molecule. As a result, the electron density on the carbon atom is reduced, making it less susceptible to nucleophilic attack.
**Comparison with Alkyl Halides**
In contrast, alkyl halides do not possess a conjugated system of π electrons and, therefore, lack resonance stabilization. The electron density on the carbon atom in alkyl halides is higher, making them more prone to nucleophilic attack.
**Other Factors**
While resonance stabilization is the primary reason for the decreased reactivity of aryl halides towards nucleophilic substitution reactions, other factors such as the larger carbon-halogen bond length and the formation of less stable carbonium ions also contribute to their lower reactivity. However, these factors are not as significant as the resonance stabilization effect.
**Conclusion**
In conclusion, aryl halides are less reactive towards nucleophilic substitution reactions compared to alkyl halides due to their resonance stabilization effect. The delocalization of electrons in the conjugated π system of the aromatic ring reduces the electron density on the carbon atom, making it less susceptible to nucleophilic attack.
Vapours of an alcohol were passed over red hot copper. It gave an olefin. The alcohol is
- a)Primary
- b) Secondary
- c) Tertiary
- d)None
Correct answer is option 'C'. Can you explain this answer?
a)
Primary
b)
Secondary
c)
Tertiary
d)
None
|
|
Aniruddha Tayade answered |
Tertiary alcohol when react with copper at 573Kdehydration takes place and alkene (olefin) is formed while primary and secondary alcohol on reaction gives aldehyde and ketone by dehydrogenation. Hence opt C is correct
The vapour pressure of a solvent decreased by 10mmHg when a nonvolatile solute was added to the solvent. The mole fraction of the solute in the solution
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm Hg- a)0.8
- b) 0.6
- c) 0.4
- d)0.2
Correct answer is option 'B'. Can you explain this answer?
is 0.2. What would be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm Hg
a)
0.8
b)
0.6
c)
0.4
d)
0.2
|
|
Harshana K A answered |

The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure?
- a)Thermoplastics
- b)Thermosetting polymers
- c)Polythylene
- d)Nylon-66
Correct answer is option 'A'. Can you explain this answer?
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
Which of the following have usually a linear structure?
a)
Thermoplastics
b)
Thermosetting polymers
c)
Polythylene
d)
Nylon-66
|
|
Muraad answered |
Thermoplastics possess a linear arrangement of atoms . They have covalent bonds between monomers and weak vander waal interaction between monomer chains. Besides they're synthesized by addition polymerization. They are lower in mol. wt than thermosetting polymers and can be remoulded into various configurations on heating.
The moleucular weight of NaCl (degree of dissociation= x) determined by the osmotic pressure method, is found to be different from its actual molecular wieght (M). Which of the following relationship is correct ?- a)Obs mol wt. = (1+ x)M
- b)M = (1+ x)×Obs mol. wt.
- c)Obs mol wt. = x×M
- d)M = x×obs mol.wt
Correct answer is option 'B'. Can you explain this answer?
a)
Obs mol wt. = (1+ x)M
b)
M = (1+ x)×Obs mol. wt.
c)
Obs mol wt. = x×M
d)
M = x×obs mol.wt
|
|
Shruti Yadav answered |
Explanation:
The osmotic pressure method is used to determine the molecular weight of a solute (in this case, NaCl) by measuring the pressure required to prevent the flow of water through a semipermeable membrane. The molecular weight obtained by this method is known as the observed molecular weight.
However, in reality, NaCl does not exist as a single molecule in solution, but rather as ions (Na+ and Cl-) that are dissociated from each other. The extent of dissociation is given by the degree of dissociation (x).
The actual molecular weight of NaCl is M, which is the sum of the atomic weights of sodium and chlorine.
Now, the relationship between the observed molecular weight and the actual molecular weight can be described as follows:
- The observed molecular weight is equal to the actual molecular weight multiplied by (1 - x). This is because the observed molecular weight is lower than the actual molecular weight due to the presence of dissociated ions that contribute to the osmotic pressure. Thus, the formula is:
Observed molecular weight = (1 - x) x Actual molecular weight
- Therefore, the correct option is B, which states that M = (1 - x) x Observed molecular weight.
The osmotic pressure method is used to determine the molecular weight of a solute (in this case, NaCl) by measuring the pressure required to prevent the flow of water through a semipermeable membrane. The molecular weight obtained by this method is known as the observed molecular weight.
However, in reality, NaCl does not exist as a single molecule in solution, but rather as ions (Na+ and Cl-) that are dissociated from each other. The extent of dissociation is given by the degree of dissociation (x).
The actual molecular weight of NaCl is M, which is the sum of the atomic weights of sodium and chlorine.
Now, the relationship between the observed molecular weight and the actual molecular weight can be described as follows:
- The observed molecular weight is equal to the actual molecular weight multiplied by (1 - x). This is because the observed molecular weight is lower than the actual molecular weight due to the presence of dissociated ions that contribute to the osmotic pressure. Thus, the formula is:
Observed molecular weight = (1 - x) x Actual molecular weight
- Therefore, the correct option is B, which states that M = (1 - x) x Observed molecular weight.
The utility of the polymers in various fields is due to their mechanical properties like tensile strength , elasticity, toughness etc.These properties mainly depend upon intermolecular forces like van der Waal’s forces and hydrogen bonding operating in polymer molecules. Polymers have been classified on this
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
The molecular forces of attraction are weakest in
- a)elastomers
- b)fibres
- c) thermoplastics
- d)thermosetting polymers
Correct answer is option 'A'. Can you explain this answer?
basis,e.g.(1) Elastomers (2) Thermoplacstics (4)Thermosetting. Hence
The molecular forces of attraction are weakest in
a)
elastomers
b)
fibres
c)
thermoplastics
d)
thermosetting polymers
|
|
Ameya Chakraborty answered |
Understanding Polymer Types
Polymers can be classified based on their mechanical properties and the intermolecular forces that hold their molecular chains together. The three main categories include elastomers, thermoplastics, and thermosetting polymers.
Elastomers: Weak Intermolecular Forces
- Elastomers are known for their exceptional elasticity and flexibility.
- The intermolecular forces in elastomers, primarily van der Waals forces and weak hydrogen bonding, are relatively weak.
- This allows elastomers to stretch and return to their original shape, making them ideal for applications like rubber bands and tires.
Thermoplastics: Moderate Intermolecular Forces
- Thermoplastics possess stronger intermolecular forces compared to elastomers.
- These materials can be heated and reshaped multiple times without significant chemical change, due to more robust van der Waals forces and some hydrogen bonding.
- Common examples include polyethylene and polystyrene.
Thermosetting Polymers: Strong Intermolecular Forces
- Thermosetting polymers have the strongest intermolecular forces among the three types.
- They undergo a chemical change during the curing process, leading to a rigid structure that cannot be remolded.
- Examples include epoxy resins and phenolic resins, which showcase high strength and thermal stability.
Conclusion: Why Elastomers Have the Weakest Forces
- The key reason elastomers have the weakest intermolecular forces is their design, which prioritizes flexibility and stretchability.
- This flexibility is essential for their applications, allowing them to absorb energy and deform under stress. Thus, the correct answer is option 'A.'
Polymers can be classified based on their mechanical properties and the intermolecular forces that hold their molecular chains together. The three main categories include elastomers, thermoplastics, and thermosetting polymers.
Elastomers: Weak Intermolecular Forces
- Elastomers are known for their exceptional elasticity and flexibility.
- The intermolecular forces in elastomers, primarily van der Waals forces and weak hydrogen bonding, are relatively weak.
- This allows elastomers to stretch and return to their original shape, making them ideal for applications like rubber bands and tires.
Thermoplastics: Moderate Intermolecular Forces
- Thermoplastics possess stronger intermolecular forces compared to elastomers.
- These materials can be heated and reshaped multiple times without significant chemical change, due to more robust van der Waals forces and some hydrogen bonding.
- Common examples include polyethylene and polystyrene.
Thermosetting Polymers: Strong Intermolecular Forces
- Thermosetting polymers have the strongest intermolecular forces among the three types.
- They undergo a chemical change during the curing process, leading to a rigid structure that cannot be remolded.
- Examples include epoxy resins and phenolic resins, which showcase high strength and thermal stability.
Conclusion: Why Elastomers Have the Weakest Forces
- The key reason elastomers have the weakest intermolecular forces is their design, which prioritizes flexibility and stretchability.
- This flexibility is essential for their applications, allowing them to absorb energy and deform under stress. Thus, the correct answer is option 'A.'
For the reaction 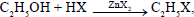 the order of reactivity is
the order of reactivity is - a)HI>HCl>HBr
- b)HI>HBr>HCl
- c)HCl>HBr>HI
- d)HBr>HI>HCl
Correct answer is option 'B'. Can you explain this answer?
a)
HI>HCl>HBr
b)
HI>HBr>HCl
c)
HCl>HBr>HI
d)
HBr>HI>HCl
|
|
Sravnikotesh Sravnikotes answered |
Longer the bond length,lesser will be dissociation energy and hence,more reactivity
Among halogen acids bond length increases from HCl to HI.
Therefore order of reactivity of halogen acids toward alcohol is
HI>HBr>HCl
Among halogen acids bond length increases from HCl to HI.
Therefore order of reactivity of halogen acids toward alcohol is
HI>HBr>HCl
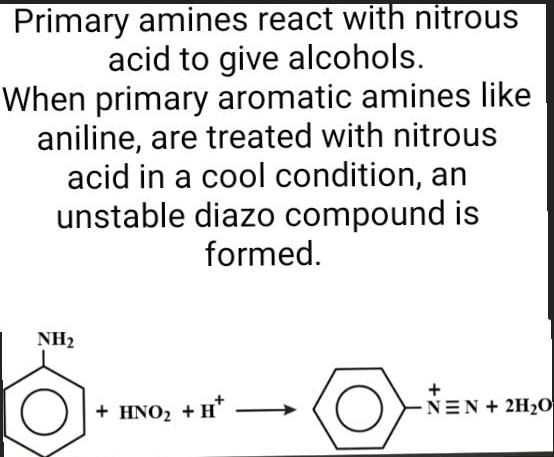
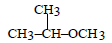
Propene is allowed to react with HI. The product (A) is then treated with 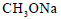 to give a new product (B)
to give a new product (B)
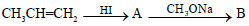
- a)
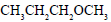
- b)
- c)
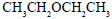
- d)
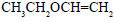
Correct answer is option 'B'. Can you explain this answer?
Propene is allowed to react with HI. The product (A) is then treated with 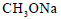 to give a new product (B)
to give a new product (B)
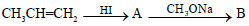
a)
b)
c)
d)
|
|
Dev Kumar answered |
Propene reacts with HI by electrophilic addition reaction to form (A) 2-iodo propane. then 2-iodo propane reacts with sodium methoxide by Williamson ether synthesis reaction to form (B) 2-methoxy propane....
so ans in option (b)
so ans in option (b)
Which of the following statements about phenol is correct?- a)It is a stronger base than ammonia
- b)It is a stronger acid than carbonic acid
- c)It is a weaker acid than carbonic acid
- d)It is neutral to litmus
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about phenol is correct?
a)
It is a stronger base than ammonia
b)
It is a stronger acid than carbonic acid
c)
It is a weaker acid than carbonic acid
d)
It is neutral to litmus
|
|
Janani Chopra answered |
Phenol as an Acid
- Phenol is a weak acid compared to carbonic acid.
- Phenol has a pKa value of around 9.95, indicating that it is a weaker acid than carbonic acid which has a pKa value of around 6.37.
- The lower the pKa value, the stronger the acid. Since carbonic acid has a lower pKa value than phenol, it is a stronger acid.
Phenol as a Base
- Phenol is not a base but rather an acidic compound.
- It does not have the ability to donate a proton to another molecule, which is a characteristic of bases.
- Ammonia, on the other hand, is a weak base as it can accept a proton to form its conjugate acid.
Phenol's Reaction with Litmus
- Phenol is slightly acidic in nature and can turn blue litmus paper red.
- Litmus paper turns red in acidic solutions and blue in basic solutions.
- Therefore, phenol would be able to change the color of litmus paper, indicating its acidic nature.
In conclusion, the correct statement is that phenol is a weaker acid compared to carbonic acid. It is not a base like ammonia and can exhibit acidic properties by changing the color of litmus paper.
- Phenol is a weak acid compared to carbonic acid.
- Phenol has a pKa value of around 9.95, indicating that it is a weaker acid than carbonic acid which has a pKa value of around 6.37.
- The lower the pKa value, the stronger the acid. Since carbonic acid has a lower pKa value than phenol, it is a stronger acid.
Phenol as a Base
- Phenol is not a base but rather an acidic compound.
- It does not have the ability to donate a proton to another molecule, which is a characteristic of bases.
- Ammonia, on the other hand, is a weak base as it can accept a proton to form its conjugate acid.
Phenol's Reaction with Litmus
- Phenol is slightly acidic in nature and can turn blue litmus paper red.
- Litmus paper turns red in acidic solutions and blue in basic solutions.
- Therefore, phenol would be able to change the color of litmus paper, indicating its acidic nature.
In conclusion, the correct statement is that phenol is a weaker acid compared to carbonic acid. It is not a base like ammonia and can exhibit acidic properties by changing the color of litmus paper.
Dry air is bubbled successively through (i) a solution,(ii) its solvant, and (iii) through 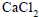 The lowering of vapour pressure of the solvant due to the addition
The lowering of vapour pressure of the solvant due to the addition
of solute is equal to - a)

- b)
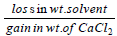
- c)
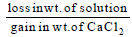
- d)
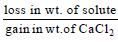
Correct answer is option 'A'. Can you explain this answer?
of solute is equal to
a)
b)
c)
d)

|
Ram Cf answered |
Quick money loan customer helpline number 9861198701//8400052097//all enquiry repayment quick money loan customer helpline number 9861198701//8400052097//all enquiry repayment
The molal depression constant for water is 1.86. The depression constant for 100g 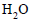 is
is - a)1.86
- b)18.6
- c)0.186
- d) 186
Correct answer is option 'A'. Can you explain this answer?
a)
1.86
b)
18.6
c)
0.186
d)
186

|
Rishika Singh answered |
Because it depend on nature of solvent not on solute
Chapter doubts & questions for Chemistry: Topic-wise Test - NEET Mock Test Series - Updated 2026 Pattern 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry: Topic-wise Test - NEET Mock Test Series - Updated 2026 Pattern in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup