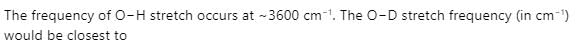All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Bioinorganic Chemistry for Chemistry Exam
The reduction of nitrogen to ammonia, carried out by the enzyme nitrogenase, needs:- a)2 electrons
- b)4 electrons
- c)6 electrons
- d)8 electrons
Correct answer is option 'D'. Can you explain this answer?
The reduction of nitrogen to ammonia, carried out by the enzyme nitrogenase, needs:
a)
2 electrons
b)
4 electrons
c)
6 electrons
d)
8 electrons
|
|
Pooja Choudhury answered |
The reduction of N2 by
this enzyme involves the transient interaction of two component proteins, designated the Fe protein and the MoFe protein, and minimally requires sixteen MgATP, eight protons, and eight electrons.
this enzyme involves the transient interaction of two component proteins, designated the Fe protein and the MoFe protein, and minimally requires sixteen MgATP, eight protons, and eight electrons.
The ligand system present in vitamin B12 is:- a)Porphyrin
- b)Corrin
- c)Phthalocyanine
- d)Crown ether
Correct answer is option 'B'. Can you explain this answer?
The ligand system present in vitamin B12 is:
a)
Porphyrin
b)
Corrin
c)
Phthalocyanine
d)
Crown ether
|
|
Aditya Deshmukh answered |
The core of the molecule vitamin B12 (cobalamin) is a corrin structure (depicted in red) with at its center a cobalt ion. One of the nitrogen atoms on the imidazole is a fifth nitrogen ligand for the cobalt atom.
The bio logical functions of carbonic anhydrase and Carboxypept idase A, respectively, are- a)Interconversion of CO2 and carbonates, and hydrolysis of pept ide bond.
- b)Gene regulation and interconversion of CO2 and carbonates
- c)Gene regulation and hydrolysis of peptide bond.
- d)Interconversion of CO2 and carbonates and gene regulation.
Correct answer is option 'A'. Can you explain this answer?
The bio logical functions of carbonic anhydrase and Carboxypept idase A, respectively, are
a)
Interconversion of CO2 and carbonates, and hydrolysis of pept ide bond.
b)
Gene regulation and interconversion of CO2 and carbonates
c)
Gene regulation and hydrolysis of peptide bond.
d)
Interconversion of CO2 and carbonates and gene regulation.

|
Priya Kaushik answered |
And is option a...,u can read the function of these in any book they will be mention specifically
In photosynthesis, the predominant metal present in the reaction centre of photo –system II is:- a)Zn
- b)Cu
- c)Mn
- d)Fe
Correct answer is option 'C'. Can you explain this answer?
In photosynthesis, the predominant metal present in the reaction centre of photo –system II is:
a)
Zn
b)
Cu
c)
Mn
d)
Fe

|
Anushka Chavan answered |
Metals exert important functions in the chloroplast of plants, where they act as cofactors and catalysts in the photosynthetic electron transport chain. In particular, manganese (Mn) has a key function because of its indispensable role in the water-splitting reaction of photosystem II (PSII).
In biological systems, the metal ions involved in electron transport are:- a)Na+ and K+
- b)Zn2+ and Mg2+
- c)Ca2+ and Mg2+
- d)Cu2+ and Fe2+
Correct answer is option 'D'. Can you explain this answer?
In biological systems, the metal ions involved in electron transport are:
a)
Na+ and K+
b)
Zn2+ and Mg2+
c)
Ca2+ and Mg2+
d)
Cu2+ and Fe2+

|
Rishabh Mehta answered |
Metal Ions in Electron Transport
Metal ions play a crucial role in electron transport in biological systems. They serve as electron carriers and are involved in redox reactions that generate energy. The correct option for the metal ions involved in electron transport is D, which is Cu2 and Fe2.
Copper (Cu2)
Copper ions are involved in electron transport in the electron transport chain of mitochondria. They are required for the activity of cytochrome c oxidase, which is the enzyme that catalyzes the final step in the electron transport chain, the reduction of oxygen to water. Copper ions are also involved in the formation of superoxide dismutase (SOD), an enzyme that protects cells against oxidative damage.
Iron (Fe2)
Iron ions are also involved in electron transport in the electron transport chain. They are required for the activity of iron-sulfur proteins, which are involved in transferring electrons between complexes in the electron transport chain. Iron-sulfur proteins are also involved in other redox reactions in cells.
Conclusion
In conclusion, copper and iron ions are the metal ions involved in electron transport in biological systems. They are essential for the activity of enzymes and proteins that are involved in redox reactions that generate energy and protect cells against oxidative damage.
Metal ions play a crucial role in electron transport in biological systems. They serve as electron carriers and are involved in redox reactions that generate energy. The correct option for the metal ions involved in electron transport is D, which is Cu2 and Fe2.
Copper (Cu2)
Copper ions are involved in electron transport in the electron transport chain of mitochondria. They are required for the activity of cytochrome c oxidase, which is the enzyme that catalyzes the final step in the electron transport chain, the reduction of oxygen to water. Copper ions are also involved in the formation of superoxide dismutase (SOD), an enzyme that protects cells against oxidative damage.
Iron (Fe2)
Iron ions are also involved in electron transport in the electron transport chain. They are required for the activity of iron-sulfur proteins, which are involved in transferring electrons between complexes in the electron transport chain. Iron-sulfur proteins are also involved in other redox reactions in cells.
Conclusion
In conclusion, copper and iron ions are the metal ions involved in electron transport in biological systems. They are essential for the activity of enzymes and proteins that are involved in redox reactions that generate energy and protect cells against oxidative damage.
The correct set of the bio logically essent ial elements, is:- a)Fe, Mo, Cu, Zn
- b)Fe, Cu, Co, Ru
- c)Cu, Mn, Zn, Ag
- d)Fe, Ru, Zn, Mg
Correct answer is option 'A'. Can you explain this answer?
The correct set of the bio logically essent ial elements, is:
a)
Fe, Mo, Cu, Zn
b)
Fe, Cu, Co, Ru
c)
Cu, Mn, Zn, Ag
d)
Fe, Ru, Zn, Mg
|
|
Pooja Choudhury answered |
- Cooper, Zinc, molybdenum and iron are considered as biologically essential elements.
- The essential mineral elements are: calcium, magnesium, sulfur, boron, chlorine, iron, manganese, zinc, copper, Nitrogen, phosphorus, potassium, molybdenum, and nickel.
- Essential elements are those elements that are required by organisms for healthy growth.
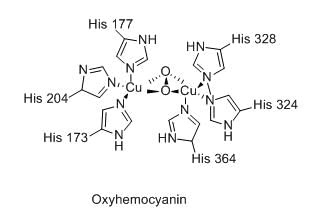
In bacterial ruberdoxin, the number of iron atoms, sulfur bridges and cysteine ligands are:
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
In bacterial ruberdoxin, the number of iron atoms, sulfur bridges and cysteine ligands are:
a)
a
b)
b
c)
c
d)
d
|
|
Ashutosh Pandey answered |
Any options aren't correct..
number of Fe is one, sulfur bridge 4 and cystine 4 are correct.....
number of Fe is one, sulfur bridge 4 and cystine 4 are correct.....
Deoxy-Hemocyanin is:- a)Heme protein and paramagnetic
- b)Colorless and diamagnetic
- c)O2 storage and paramagnetic
- d)Blue colored and diamangnetic
Correct answer is option 'B'. Can you explain this answer?
Deoxy-Hemocyanin is:
a)
Heme protein and paramagnetic
b)
Colorless and diamagnetic
c)
O2 storage and paramagnetic
d)
Blue colored and diamangnetic

|
Anirban Khanna answered |
Hemocyanin is blue pigment which contains Cu in (I) oxidation state.Since it is in deoxy form it is colorless and also diamagnetic (d10) configuration.
The oxidation state of iron in methemoglobin is:- a)Three
- b)Two
- c)Four
- d)Zero
Correct answer is option 'A'. Can you explain this answer?
The oxidation state of iron in methemoglobin is:
a)
Three
b)
Two
c)
Four
d)
Zero
|
|
Pooja Choudhury answered |
- Methemoglobin is hemoglobin in the form of metalloprotein, in which the iron in the heme group is in the Fe³⁺ state, not the Fe²⁺ of normal hemoglobin.
- Methemoglobin cannot bind oxygen, which means it cannot carry oxygen to tissues.
- It is a bluish chocolate-brown in colour.
Hemoglobin is an oxygen carrying. The correct statement about oxy–hemoglobin is that:- a)The metal is low–spin in +3 oxidation state while dioxygen is in O2- form.
- b)The metal is high–spin in +3 oxidat ion state while dioxygen is in O2- form.
- c)The metal is low–spin in +3 oxidation state while dioxygen is in neutral form.
- d)The metal is high–spin in +3 oxidation state while dioxygen is in neutral form.
Correct answer is option 'A'. Can you explain this answer?
Hemoglobin is an oxygen carrying. The correct statement about oxy–hemoglobin is that:
a)
The metal is low–spin in +3 oxidation state while dioxygen is in O2- form.
b)
The metal is high–spin in +3 oxidat ion state while dioxygen is in O2- form.
c)
The metal is low–spin in +3 oxidation state while dioxygen is in neutral form.
d)
The metal is high–spin in +3 oxidation state while dioxygen is in neutral form.

|
Bijoy Kapoor answered |
Hemoglobin (Heme + Globin) The protein hemoglobin is a molecule which is responsible for carrying almost all of the oxygen in the blood. ... The heme group is composed of a porphyrin ring which contains an iron (Fe) atom in its center. Normally, the Fe is in the +2 redox state (ferrous) and can reversibly bind oxygen.
High dose of dietary supplement ZnSO4 for the cure of Zn deficiency:- a)Reduce myoglobin.
- b)Increases iron level in blood.
- c)Increase copper level in brain.
- d)Reduce copper, iron and calcium levels in body.
Correct answer is option 'D'. Can you explain this answer?
High dose of dietary supplement ZnSO4 for the cure of Zn deficiency:
a)
Reduce myoglobin.
b)
Increases iron level in blood.
c)
Increase copper level in brain.
d)
Reduce copper, iron and calcium levels in body.

|
Anshika Chavan answered |
The correct answer is D. High dose of dietary supplement ZnSO4 for the cure of Zn deficiency results in the reduction of copper, iron, and calcium levels in the body. This is because of the following reasons:
Zinc and Copper:
- Zinc and copper have an inverse relationship in the body. High doses of zinc can interfere with copper absorption and utilization in the body.
- Zinc competes with copper for binding sites on proteins and enzymes, which can lead to a decrease in copper levels.
- Copper is an essential element for many bodily functions, including the formation of red blood cells and the maintenance of the immune system. A reduction in copper levels can lead to anemia and weakened immunity.
Zinc and Iron:
- Zinc and iron also have an inverse relationship in the body. High doses of zinc can interfere with iron absorption and utilization in the body.
- Zinc can bind to iron in the gastrointestinal tract, preventing its absorption.
- Iron is essential for the formation of hemoglobin, the protein in red blood cells that carries oxygen throughout the body. A reduction in iron levels can lead to anemia.
Zinc and Calcium:
- High doses of zinc can also interfere with calcium absorption and utilization in the body.
- Zinc can bind to calcium in the gastrointestinal tract, preventing its absorption.
- Calcium is essential for bone health and muscle function. A reduction in calcium levels can lead to osteoporosis and muscle weakness.
In summary, high doses of dietary supplement ZnSO4 for the cure of Zn deficiency can lead to a reduction in copper, iron, and calcium levels in the body, which can have negative health consequences. Therefore, it is important to take dietary supplements under the guidance of a healthcare professional to avoid any potential adverse effects.
Zinc and Copper:
- Zinc and copper have an inverse relationship in the body. High doses of zinc can interfere with copper absorption and utilization in the body.
- Zinc competes with copper for binding sites on proteins and enzymes, which can lead to a decrease in copper levels.
- Copper is an essential element for many bodily functions, including the formation of red blood cells and the maintenance of the immune system. A reduction in copper levels can lead to anemia and weakened immunity.
Zinc and Iron:
- Zinc and iron also have an inverse relationship in the body. High doses of zinc can interfere with iron absorption and utilization in the body.
- Zinc can bind to iron in the gastrointestinal tract, preventing its absorption.
- Iron is essential for the formation of hemoglobin, the protein in red blood cells that carries oxygen throughout the body. A reduction in iron levels can lead to anemia.
Zinc and Calcium:
- High doses of zinc can also interfere with calcium absorption and utilization in the body.
- Zinc can bind to calcium in the gastrointestinal tract, preventing its absorption.
- Calcium is essential for bone health and muscle function. A reduction in calcium levels can lead to osteoporosis and muscle weakness.
In summary, high doses of dietary supplement ZnSO4 for the cure of Zn deficiency can lead to a reduction in copper, iron, and calcium levels in the body, which can have negative health consequences. Therefore, it is important to take dietary supplements under the guidance of a healthcare professional to avoid any potential adverse effects.
Oxymyoglobin Mb(O2) and oxyhaemoglobin Hb(O2)4, respectively, are:- a)Paramagnetic and paramagnetic.
- b)Diamagnetic and diamagnetic.
- c)Paramagnetic and diamagnetic.
- d)Diamagnetic and paramagnetic.
Correct answer is option 'B'. Can you explain this answer?
Oxymyoglobin Mb(O2) and oxyhaemoglobin Hb(O2)4, respectively, are:
a)
Paramagnetic and paramagnetic.
b)
Diamagnetic and diamagnetic.
c)
Paramagnetic and diamagnetic.
d)
Diamagnetic and paramagnetic.

|
Akanksha Choudhary answered |
Diamagnetic and Diamagnetic
Oxymyoglobin Mb(O2) and oxyhaemoglobin Hb(O2)4 are both diamagnetic.
Explanation:
1. Diamagnetic and Paramagnetic
Diamagnetic substances have all their electrons paired up and do not exhibit any magnetic properties. On the other hand, paramagnetic substances have unpaired electrons and are attracted to a magnetic field.
2. Oxyhaemoglobin and Oxymyoglobin
Oxyhaemoglobin and oxymyoglobin are both forms of hemoglobin that carry oxygen. Oxyhaemoglobin is present in red blood cells and is responsible for carrying oxygen from the lungs to the rest of the body. Oxymyoglobin is present in muscle tissue and is responsible for storing oxygen that can be used during muscle activity.
3. Magnetic Properties
Both oxyhaemoglobin and oxymyoglobin have diamagnetic properties. This is because they do not have any unpaired electrons and do not exhibit any magnetic properties in the presence of a magnetic field.
4. Conclusion
In conclusion, oxyhaemoglobin Hb(O2)4 and oxymyoglobin Mb(O2) are both diamagnetic substances as they do not have any unpaired electrons and do not exhibit any magnetic properties.
Oxymyoglobin Mb(O2) and oxyhaemoglobin Hb(O2)4 are both diamagnetic.
Explanation:
1. Diamagnetic and Paramagnetic
Diamagnetic substances have all their electrons paired up and do not exhibit any magnetic properties. On the other hand, paramagnetic substances have unpaired electrons and are attracted to a magnetic field.
2. Oxyhaemoglobin and Oxymyoglobin
Oxyhaemoglobin and oxymyoglobin are both forms of hemoglobin that carry oxygen. Oxyhaemoglobin is present in red blood cells and is responsible for carrying oxygen from the lungs to the rest of the body. Oxymyoglobin is present in muscle tissue and is responsible for storing oxygen that can be used during muscle activity.
3. Magnetic Properties
Both oxyhaemoglobin and oxymyoglobin have diamagnetic properties. This is because they do not have any unpaired electrons and do not exhibit any magnetic properties in the presence of a magnetic field.
4. Conclusion
In conclusion, oxyhaemoglobin Hb(O2)4 and oxymyoglobin Mb(O2) are both diamagnetic substances as they do not have any unpaired electrons and do not exhibit any magnetic properties.
The changes (from A–D given below) which occur when O2 binds to hemerythrin are:
(A) One ion atom is oxidized.
(B) Both the iron atoms are oxidized.
(C) O2 binds to one iron atom and is also hydrogen bonds.
(D) O2 binds to both the iron atoms and is also hydrogen bonded.- a)B and C
- b)B and D
- c)A and D
- d)A and C
Correct answer is option 'A'. Can you explain this answer?
The changes (from A–D given below) which occur when O2 binds to hemerythrin are:
(A) One ion atom is oxidized.
(B) Both the iron atoms are oxidized.
(C) O2 binds to one iron atom and is also hydrogen bonds.
(D) O2 binds to both the iron atoms and is also hydrogen bonded.
(A) One ion atom is oxidized.
(B) Both the iron atoms are oxidized.
(C) O2 binds to one iron atom and is also hydrogen bonds.
(D) O2 binds to both the iron atoms and is also hydrogen bonded.
a)
B and C
b)
B and D
c)
A and D
d)
A and C

|
Aditi Basak answered |
The changes that occur when O2 binds to hemerythrin are:
(A) One ion atom is oxidized.
Explanation:
Hemerythrin is a non-heme iron protein found in marine invertebrates that functions as an oxygen carrier. It contains two iron atoms that can bind to oxygen molecules. When O2 binds to hemerythrin, one of the iron atoms is oxidized from the ferrous (Fe2+) to the ferric (Fe3+) state, resulting in the formation of a peroxide bridge between the two iron atoms. This oxidation reaction is shown below:
Fe2+ + O2 → Fe3+ - O2-
The peroxide bridge stabilizes the bound O2 molecule and prevents it from dissociating from the iron atoms.
In addition to the oxidation of one iron atom, there is no hydrogen bonding involved in the binding of O2 to hemerythrin. The binding of O2 to hemerythrin is purely based on the coordination of the O2 molecule with the iron atoms.
Therefore, the correct answer is option 'A' - One ion atom is oxidized.
(A) One ion atom is oxidized.
Explanation:
Hemerythrin is a non-heme iron protein found in marine invertebrates that functions as an oxygen carrier. It contains two iron atoms that can bind to oxygen molecules. When O2 binds to hemerythrin, one of the iron atoms is oxidized from the ferrous (Fe2+) to the ferric (Fe3+) state, resulting in the formation of a peroxide bridge between the two iron atoms. This oxidation reaction is shown below:
Fe2+ + O2 → Fe3+ - O2-
The peroxide bridge stabilizes the bound O2 molecule and prevents it from dissociating from the iron atoms.
In addition to the oxidation of one iron atom, there is no hydrogen bonding involved in the binding of O2 to hemerythrin. The binding of O2 to hemerythrin is purely based on the coordination of the O2 molecule with the iron atoms.
Therefore, the correct answer is option 'A' - One ion atom is oxidized.
Superoxide dismutase contains the metal ions:- a)Zn (II) and Ni (II)
- b)Cu (II) and Zn (II)
- c)Ni (II) and Co (III)
- d)Cu (II) and Fe (III)
Correct answer is option 'B'. Can you explain this answer?
Superoxide dismutase contains the metal ions:
a)
Zn (II) and Ni (II)
b)
Cu (II) and Zn (II)
c)
Ni (II) and Co (III)
d)
Cu (II) and Fe (III)
|
|
Pooja Choudhury answered |
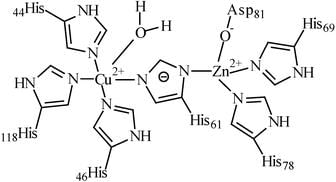
Chemical Structure of Superoxide Dismutase


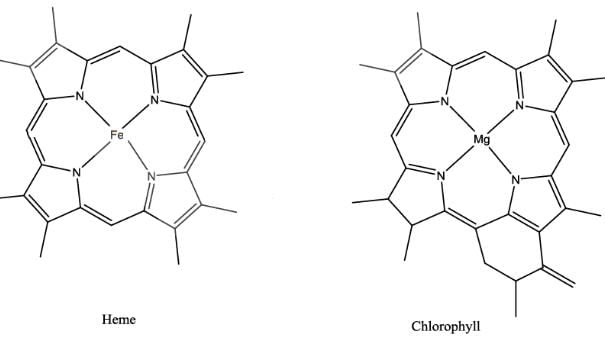
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:- a) (i) > (iii) > (ii)
- b)(i) > (ii) > (iii)
- c)(iii) > (i) > (ii)
- d)(ii) ≈ (i) > (iii)
Correct answer is option 'A'. Can you explain this answer?
The extent of π electron conjugation in macrocyclic rings of (i) heme, (ii) coenzyme B12 and (iii) chlorophyll follows the order:
a)
(i) > (iii) > (ii)
b)
(i) > (ii) > (iii)
c)
(iii) > (i) > (ii)
d)
(ii) ≈ (i) > (iii)

|
Om Desai answered |
Least conjugation is present in Vitamin B12 as one side of the corrin ring doesn’t have methylene linkages. In Heme, there is extensive conjugation of π electron (11 π ) as compared to that of chlorophyll.( 10π )
The number of oxygen molecule(s) that a molecule of hemerythrin can transport is:- a)One
- b)Two
- c)Three
- d)Four
Correct answer is option 'A'. Can you explain this answer?
The number of oxygen molecule(s) that a molecule of hemerythrin can transport is:
a)
One
b)
Two
c)
Three
d)
Four

|
Swara Reddy answered |
Hemerythrin is a metalloprotein that plays an important role in transporting oxygen in some invertebrates. It contains iron atoms that can bind to oxygen molecules. The number of oxygen molecule(s) that a molecule of hemerythrin can transport is:
One
Explanation:
Hemerythrin has two iron atoms that can each bind to one oxygen molecule, but the molecule as a whole can only transport one oxygen molecule at a time. This is because the two iron atoms are separated by a distance that is too great to allow both to bind to oxygen simultaneously. Therefore, each hemerythrin molecule can only transport one oxygen molecule at a time.
In summary, the number of oxygen molecule(s) that a molecule of hemerythrin can transport is one.
One
Explanation:
Hemerythrin has two iron atoms that can each bind to one oxygen molecule, but the molecule as a whole can only transport one oxygen molecule at a time. This is because the two iron atoms are separated by a distance that is too great to allow both to bind to oxygen simultaneously. Therefore, each hemerythrin molecule can only transport one oxygen molecule at a time.
In summary, the number of oxygen molecule(s) that a molecule of hemerythrin can transport is one.
Amongst the following, the group that is bound to the metal ion in cenzyme B12 is:- a)Methyl
- b)Cyanide
- c)Adenosyl
- d)Hydroxyl
Correct answer is option 'C'. Can you explain this answer?
Amongst the following, the group that is bound to the metal ion in cenzyme B12 is:
a)
Methyl
b)
Cyanide
c)
Adenosyl
d)
Hydroxyl

|
Bijoy Kapoor answered |
Coenzymes are small organic molecules that link to enzymes and whose presence is essential to the activity of those enzymes. Coenzymes belong to the larger group called cofactors, which also includes metal ions; cofactor is the more general term for small molecules required for the activity of their associated enzymes.
Many coenzymes are derived from vitamins . Table 1 lists vitamins, the coenzymes derived from them, the type of reactions in which they participate, and the class of coenzyme.
Prosthetic groups are tightly bound to enzymes and participate in the catalytic cycles of enzymes. Like any catalyst , an enzyme–prosthetic group complex undergoes changes during the reaction, but before it can catalyze another reaction, it must return to its original state.
Flavin adenine dinucleotide (FAD) is a prosthetic group that participates in several intracellular oxidation -reduction reactions. During the catalytic cycle of the enzyme succinate dehydrogenase, FAD accepts two electrons from succinate, yielding fumarate as a product. Because FAD is tightly bound to the enzyme, the reaction is sometimes shown this way
succinate + E–FAD → fumarate + E–FADH 2
where E–FAD stands for the enzyme tightly bound to the FAD prosthetic group. In this reaction the coenzyme FAD is reduced to FADH 2 and remains tightly bound to the enzyme throughout. Before the enzyme can catalyze the oxidation of another succinate molecule, the two electrons now belonging to E–FADH 2 must be transferred to another electron acceptor, ubiquinone. The regenerated E–FAD complex can then oxidize another succinate molecule.
A well known naturally occurring organometallic compound is:- a)Vitamin B12 coenzyme.
- b)Chlorophyll.
- c)Cytochrome P–450.
- d)Myoglobin.
Correct answer is option 'A'. Can you explain this answer?
A well known naturally occurring organometallic compound is:
a)
Vitamin B12 coenzyme.
b)
Chlorophyll.
c)
Cytochrome P–450.
d)
Myoglobin.

|
Mrinalini Singh answered |
Naturally Occurring Organometallic Compound: Vitamin B12 Coenzyme
Organometallic compounds are compounds containing metal-carbon bonds. These compounds can be found in various biological systems and play an important role in many metabolic reactions. One of the most well-known naturally occurring organometallic compounds is Vitamin B12 coenzyme.
Structure of Vitamin B12 Coenzyme:
- Vitamin B12 coenzyme is also known as cobalamin. It contains a cobalt atom in its center, which is coordinated to a corrin ring.
- The corrin ring is a macrocyclic structure consisting of four pyrrole rings linked together by methylene bridges.
- The corrin ring is also coordinated to a dimethylbenzimidazole group and a nucleotide group, which can be either 5,6-dimethylbenzimidazole or 5'-deoxyadenosyl.
- The nucleotide group is important for the function of Vitamin B12 coenzyme as it allows it to participate in enzymatic reactions.
Function of Vitamin B12 Coenzyme:
- Vitamin B12 coenzyme plays a critical role in the metabolism of amino acids, fatty acids, and carbohydrates.
- It acts as a cofactor for two important enzymes: methionine synthase and methylmalonyl-CoA mutase.
- Methionine synthase is involved in the conversion of homocysteine to methionine, which is an important step in the synthesis of proteins and other molecules.
- Methylmalonyl-CoA mutase is involved in the breakdown of certain amino acids and fatty acids.
Deficiency of Vitamin B12 Coenzyme:
- Deficiency of Vitamin B12 coenzyme can lead to a number of health problems, including anemia, nerve damage, and cognitive impairment.
- It is particularly important for vegetarians and vegans to ensure that they get enough Vitamin B12 in their diet as it is primarily found in animal-based foods.
Conclusion:
Vitamin B12 coenzyme is a naturally occurring organometallic compound that plays a critical role in many metabolic reactions. Its structure contains a cobalt atom coordinated to a corrin ring, dimethylbenzimidazole group, and a nucleotide group. It acts as a cofactor for two important enzymes involved in the metabolism of amino acids, fatty acids, and carbohydrates. Deficiency of Vitamin B12 coenzyme can have serious health consequences, particularly for vegetarians and vegans.
Organometallic compounds are compounds containing metal-carbon bonds. These compounds can be found in various biological systems and play an important role in many metabolic reactions. One of the most well-known naturally occurring organometallic compounds is Vitamin B12 coenzyme.
Structure of Vitamin B12 Coenzyme:
- Vitamin B12 coenzyme is also known as cobalamin. It contains a cobalt atom in its center, which is coordinated to a corrin ring.
- The corrin ring is a macrocyclic structure consisting of four pyrrole rings linked together by methylene bridges.
- The corrin ring is also coordinated to a dimethylbenzimidazole group and a nucleotide group, which can be either 5,6-dimethylbenzimidazole or 5'-deoxyadenosyl.
- The nucleotide group is important for the function of Vitamin B12 coenzyme as it allows it to participate in enzymatic reactions.
Function of Vitamin B12 Coenzyme:
- Vitamin B12 coenzyme plays a critical role in the metabolism of amino acids, fatty acids, and carbohydrates.
- It acts as a cofactor for two important enzymes: methionine synthase and methylmalonyl-CoA mutase.
- Methionine synthase is involved in the conversion of homocysteine to methionine, which is an important step in the synthesis of proteins and other molecules.
- Methylmalonyl-CoA mutase is involved in the breakdown of certain amino acids and fatty acids.
Deficiency of Vitamin B12 Coenzyme:
- Deficiency of Vitamin B12 coenzyme can lead to a number of health problems, including anemia, nerve damage, and cognitive impairment.
- It is particularly important for vegetarians and vegans to ensure that they get enough Vitamin B12 in their diet as it is primarily found in animal-based foods.
Conclusion:
Vitamin B12 coenzyme is a naturally occurring organometallic compound that plays a critical role in many metabolic reactions. Its structure contains a cobalt atom coordinated to a corrin ring, dimethylbenzimidazole group, and a nucleotide group. It acts as a cofactor for two important enzymes involved in the metabolism of amino acids, fatty acids, and carbohydrates. Deficiency of Vitamin B12 coenzyme can have serious health consequences, particularly for vegetarians and vegans.
Among the given pH values, the O2 binding efficiency of hemoglobin is maximum at:- a)6.8
- b)7.0
- c)7.2
- d)7.4
Correct answer is option 'D'. Can you explain this answer?
Among the given pH values, the O2 binding efficiency of hemoglobin is maximum at:
a)
6.8
b)
7.0
c)
7.2
d)
7.4
|
|
Mehak Saini answered |
Affinity of oxygen increases with increase in ph ...called bohr effect
Under physiological condition, oxygen is binding to deoxyhemoglobin and deoxymyoglobin, the binding curve and its pH dependence, respectively, are:- a)Sigmoidal and pH dependent; hyperbolic and pH independent.
- b)Hyperbolic and pH independent; Sigmoidal and pH dependent.
- c)Sigmoidal and pH independent; hyperbolic and pH independent.
- d)Hyperbolic and pH dependent; Sigmoidal and pH dependent.
Correct answer is option 'A'. Can you explain this answer?
Under physiological condition, oxygen is binding to deoxyhemoglobin and deoxymyoglobin, the binding curve and its pH dependence, respectively, are:
a)
Sigmoidal and pH dependent; hyperbolic and pH independent.
b)
Hyperbolic and pH independent; Sigmoidal and pH dependent.
c)
Sigmoidal and pH independent; hyperbolic and pH independent.
d)
Hyperbolic and pH dependent; Sigmoidal and pH dependent.

|
Swara Reddy answered |
Overview of Hemoglobin and Myoglobin Binding
Under physiological conditions, the binding of oxygen to deoxyhemoglobin and deoxymyoglobin exhibits distinct characteristics due to their structural and functional differences.
Oxygen Binding Characteristics
- Deoxyhemoglobin:
- Exhibits a sigmoidal binding curve due to cooperative binding, meaning the binding of one oxygen molecule increases the likelihood of others binding.
- This cooperativity allows hemoglobin to effectively pick up oxygen in the lungs and release it in tissues.
- The binding is pH dependent, influenced by the Bohr effect, where lower pH (higher H+ concentration) decreases oxygen affinity, facilitating oxygen release where it is needed most.
- Deoxymyoglobin:
- Displays a hyperbolic binding curve, indicating that each myoglobin molecule binds oxygen independently and does not exhibit cooperative binding.
- This allows myoglobin to effectively store oxygen in muscles for use during intense activity.
- The binding characteristics of myoglobin are generally pH independent, meaning that changes in pH do not significantly affect its oxygen affinity.
Summary of Answer
Thus, the correct answer is option 'A':
- Sigmoidal and pH dependent for deoxyhemoglobin
- Hyperbolic and pH independent for deoxymyoglobin
Understanding these differences is crucial for comprehending how oxygen transport and storage function in the body, particularly during varying physiological conditions.
Under physiological conditions, the binding of oxygen to deoxyhemoglobin and deoxymyoglobin exhibits distinct characteristics due to their structural and functional differences.
Oxygen Binding Characteristics
- Deoxyhemoglobin:
- Exhibits a sigmoidal binding curve due to cooperative binding, meaning the binding of one oxygen molecule increases the likelihood of others binding.
- This cooperativity allows hemoglobin to effectively pick up oxygen in the lungs and release it in tissues.
- The binding is pH dependent, influenced by the Bohr effect, where lower pH (higher H+ concentration) decreases oxygen affinity, facilitating oxygen release where it is needed most.
- Deoxymyoglobin:
- Displays a hyperbolic binding curve, indicating that each myoglobin molecule binds oxygen independently and does not exhibit cooperative binding.
- This allows myoglobin to effectively store oxygen in muscles for use during intense activity.
- The binding characteristics of myoglobin are generally pH independent, meaning that changes in pH do not significantly affect its oxygen affinity.
Summary of Answer
Thus, the correct answer is option 'A':
- Sigmoidal and pH dependent for deoxyhemoglobin
- Hyperbolic and pH independent for deoxymyoglobin
Understanding these differences is crucial for comprehending how oxygen transport and storage function in the body, particularly during varying physiological conditions.
Based on the behavior of the metalloenzymes, consider the following statements:(A) In the enzymes, the zinc act ivates O2 to form peroxide species.
(B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide.
(C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens.
(D) Zincion acts as a nucleophile and attacks at the peptide carbonyl.The set of correct statements is,- a)A and B
- b)B and C
- c)C and D
- d)A and D
Correct answer is option 'B'. Can you explain this answer?
Based on the behavior of the metalloenzymes, consider the following statements:
(A) In the enzymes, the zinc act ivates O2 to form peroxide species.
(B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide.
(C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens.
(D) Zincion acts as a nucleophile and attacks at the peptide carbonyl.
(B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide.
(C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens.
(D) Zincion acts as a nucleophile and attacks at the peptide carbonyl.
The set of correct statements is,
a)
A and B
b)
B and C
c)
C and D
d)
A and D

|
Pragati Sharma answered |
Metalloenzymes and the Role of Zinc Ion:
Metalloenzymes are enzymes that require a metal ion for their biological activity. Zinc ion is one such metal ion required by many enzymes. Let us consider the given statements and understand the behavior of metalloenzymes with respect to the role of zinc ion.
A) In the enzymes, the zinc activates O2 to form peroxide species:
This statement is incorrect because zinc ion does not activate O2 to form peroxide species. Instead, copper and iron ions are known to activate O2 to form peroxide species.
B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide:
This statement is correct. Many metalloenzymes require zinc ion to activate water molecules and form zinc-bound hydroxide, which further participates in catalysis.
C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens:
This statement is incorrect because zinc ion does not activate O2 to break the bonding between the two oxygens. Instead, copper and iron ions are known to activate O2 to break the O-O bond.
D) Zinc ion acts as a nucleophile and attacks at the peptide carbonyl:
This statement is incorrect because zinc ion is not known to act as a nucleophile and attack the peptide carbonyl. Instead, zinc ion is known to stabilize the transition state of the peptide bond formation.
Therefore, the correct statements are B and C, and the correct option is (b) B and C.
Metalloenzymes are enzymes that require a metal ion for their biological activity. Zinc ion is one such metal ion required by many enzymes. Let us consider the given statements and understand the behavior of metalloenzymes with respect to the role of zinc ion.
A) In the enzymes, the zinc activates O2 to form peroxide species:
This statement is incorrect because zinc ion does not activate O2 to form peroxide species. Instead, copper and iron ions are known to activate O2 to form peroxide species.
B) In the enzymes, the zinc activates H2O and provides a zinc bound hydroxide:
This statement is correct. Many metalloenzymes require zinc ion to activate water molecules and form zinc-bound hydroxide, which further participates in catalysis.
C) In the enzymes, the zinc activates O2 to break the bounding between the two oxygens:
This statement is incorrect because zinc ion does not activate O2 to break the bonding between the two oxygens. Instead, copper and iron ions are known to activate O2 to break the O-O bond.
D) Zinc ion acts as a nucleophile and attacks at the peptide carbonyl:
This statement is incorrect because zinc ion is not known to act as a nucleophile and attack the peptide carbonyl. Instead, zinc ion is known to stabilize the transition state of the peptide bond formation.
Therefore, the correct statements are B and C, and the correct option is (b) B and C.
A metal ion that replace manganese (II) ion in Mangano–proteins without changing its function, is:- a)Fe (II)
- b)Zn (II)
- c)Mg (II)
- d)Cu (II)
Correct answer is option 'C'. Can you explain this answer?
A metal ion that replace manganese (II) ion in Mangano–proteins without changing its function, is:
a)
Fe (II)
b)
Zn (II)
c)
Mg (II)
d)
Cu (II)

|
Yash Roy answered |
Metal ion Replacement in Manganoproteins:
Manganoproteins are proteins that contain manganese ions in their active site. These proteins are involved in various biochemical processes such as photosynthesis, respiration, and antioxidant defense. Sometimes, other metal ions can replace manganese ions in these proteins without changing their function.
Metal ion Replacement Options:
The metal ions that can replace manganese (II) ion in manganoproteins without changing its function are:
a) Fe (II)
b) Zn (II)
c) Mg (II)
d) Cu (II)
Correct Answer and Explanation:
The correct answer is option 'C' which is Mg (II). Magnesium ion is similar in size and electronic configuration to manganese ion, which makes it an ideal replacement. Moreover, magnesium ion is also biologically important and found in various enzymes and proteins.
Other Options Explanation:
a) Fe (II): Iron ion has a larger size and different electronic configuration than manganese ion, which makes it unsuitable for replacement. Moreover, iron ion is involved in various biological processes and can cause functional changes in manganoproteins.
b) Zn (II): Zinc ion is smaller in size than manganese ion and has a different electronic configuration, which makes it unsuitable for replacement. Moreover, zinc ion is involved in various biological processes and can cause functional changes in manganoproteins.
d) Cu (II): Copper ion has a different electronic configuration than manganese ion, which makes it unsuitable for replacement. Moreover, copper ion is involved in various biological processes and can cause functional changes in manganoproteins.
Manganoproteins are proteins that contain manganese ions in their active site. These proteins are involved in various biochemical processes such as photosynthesis, respiration, and antioxidant defense. Sometimes, other metal ions can replace manganese ions in these proteins without changing their function.
Metal ion Replacement Options:
The metal ions that can replace manganese (II) ion in manganoproteins without changing its function are:
a) Fe (II)
b) Zn (II)
c) Mg (II)
d) Cu (II)
Correct Answer and Explanation:
The correct answer is option 'C' which is Mg (II). Magnesium ion is similar in size and electronic configuration to manganese ion, which makes it an ideal replacement. Moreover, magnesium ion is also biologically important and found in various enzymes and proteins.
Other Options Explanation:
a) Fe (II): Iron ion has a larger size and different electronic configuration than manganese ion, which makes it unsuitable for replacement. Moreover, iron ion is involved in various biological processes and can cause functional changes in manganoproteins.
b) Zn (II): Zinc ion is smaller in size than manganese ion and has a different electronic configuration, which makes it unsuitable for replacement. Moreover, zinc ion is involved in various biological processes and can cause functional changes in manganoproteins.
d) Cu (II): Copper ion has a different electronic configuration than manganese ion, which makes it unsuitable for replacement. Moreover, copper ion is involved in various biological processes and can cause functional changes in manganoproteins.
Iron–sulphur clusters in bio logical systems are involved in:- a)Proton transfer
- b)Atom transfer
- c)Group transfer
- d)Electron transfer
Correct answer is option 'D'. Can you explain this answer?
Iron–sulphur clusters in bio logical systems are involved in:
a)
Proton transfer
b)
Atom transfer
c)
Group transfer
d)
Electron transfer

|
Harshitha Sharma answered |
Ironsulphur clusters in biological systems are involved in electron transfer.
- Introduction: Ironsulphur clusters are protein-bound metal cofactors that are ubiquitous in nature. They play a vital role in various enzymatic processes, including electron transfer reactions in biological systems.
- Electron transfer: Ironsulphur clusters facilitate electron transfer reactions by serving as electron carriers. They can accept or donate electrons, depending on the redox state of the cluster. Ironsulphur clusters are involved in a wide range of biological electron transfer reactions, including photosynthesis, respiration, and DNA repair.
- Structure: Ironsulphur clusters are composed of iron and sulphur atoms that are coordinated by cysteine residues in proteins. The clusters can vary in size and structure, ranging from simple [2Fe-2S] or [4Fe-4S] clusters to more complex clusters that contain additional metal ions and ligands.
- Function: The function of ironsulphur clusters in electron transfer reactions is based on their ability to transfer electrons between different redox states. This transfer of electrons is facilitated by the unique electronic properties of the iron and sulphur atoms in the cluster, which allow them to accept or donate electrons in a controlled manner.
- Conclusion: In conclusion, ironsulphur clusters are essential components of many biological systems. They play a crucial role in electron transfer reactions, which are vital for energy production, DNA repair, and other cellular processes. Understanding the structure and function of these clusters is essential for developing new therapies for various diseases and for optimizing industrial processes that rely on biological electron transfer reactions.
The cooperative binding Of O2 in hemoglobin is due to:- a)A decrease in size of iron followed by changes in the proton conformation.
- b)An increase in size of iron fo llowed by changes in the proton conformat ion.
- c)A decrease in size of iron that is not accompanied by the protein comformational changes.
- d)An increase in size of iron that is not accompanied by the protein comformational changes.
Correct answer is option 'A'. Can you explain this answer?
The cooperative binding Of O2 in hemoglobin is due to:
a)
A decrease in size of iron followed by changes in the proton conformation.
b)
An increase in size of iron fo llowed by changes in the proton conformat ion.
c)
A decrease in size of iron that is not accompanied by the protein comformational changes.
d)
An increase in size of iron that is not accompanied by the protein comformational changes.

|
Sagarika Patel answered |
Hemoglobin exhibits what we call cooperative binding, as oxygen binding increases the affinity of hemoglobin for more oxygen. Increased affinity is caused by a conformational change, or a structural change in the hemoglobin molecule.
The red color of oxyhaemoglobin is mainly due to the:- a)d–d transition
- b)Metal to ligand charge transfer transition
- c)Ligand to metal charge transfer transit ion
- d)Intra ligand π–π* transition.
Correct answer is option 'D'. Can you explain this answer?
The red color of oxyhaemoglobin is mainly due to the:
a)
d–d transition
b)
Metal to ligand charge transfer transition
c)
Ligand to metal charge transfer transit ion
d)
Intra ligand π–π* transition.

|
Vandana Gupta answered |
Each hemoglobin protein is made up subunits called hemes, which are what give blood its red color. More specifically, the hemes can bind iron molecules, and these iron molecules bind oxygen. The blood cells are red because of the interaction between iron and oxygen i.e. intra ligand π–π* transition.
When the iron is oxygenated(Fe+3), it becomes red. When the iron is deoxygenated(Fe+2), it becomes blue. This is why your veins are blue.
Among the following pair of metal ions present in Nature. The first one functions as an electron transfer agent and the second one catalyzes the hydrolysis reactions. The correct pair is:- a)Fe and Zn
- b)Mg and Fe
- c)Co and Mo
- d)Ca and Cu
Correct answer is option 'A'. Can you explain this answer?
Among the following pair of metal ions present in Nature. The first one functions as an electron transfer agent and the second one catalyzes the hydrolysis reactions. The correct pair is:
a)
Fe and Zn
b)
Mg and Fe
c)
Co and Mo
d)
Ca and Cu

|
Shivam Khanna answered |
Metal Ions in Nature
Function of Metal Ions
Metal ions play crucial roles in various biological processes. They are involved in electron transfer, catalysis, and structural stabilization.
Pair of Metal Ions
The pair of metal ions that functions as an electron transfer agent and catalyzes hydrolysis reactions are Fe and Zn.
Function of Fe
Iron (Fe) is an essential element for life and plays a crucial role in various biological processes. Fe acts as an electron transfer agent in many enzymes. For example, Fe is present in the heme group of hemoglobin and myoglobin, which are involved in oxygen transport in the body. Fe is also present in cytochromes, which are involved in electron transfer in the respiratory chain.
Function of Zn
Zinc (Zn) is also an essential element for life and plays a crucial role in various biological processes. Zn is involved in catalysis and structural stabilization. Zn is present in many enzymes, such as carbonic anhydrase, which catalyzes the reversible hydration of carbon dioxide. Zn is also present in many DNA and RNA binding proteins, where it stabilizes the structure of the molecule.
Conclusion
In conclusion, Fe and Zn are a pair of metal ions present in nature. Fe functions as an electron transfer agent, while Zn catalyzes hydrolysis reactions.
Function of Metal Ions
Metal ions play crucial roles in various biological processes. They are involved in electron transfer, catalysis, and structural stabilization.
Pair of Metal Ions
The pair of metal ions that functions as an electron transfer agent and catalyzes hydrolysis reactions are Fe and Zn.
Function of Fe
Iron (Fe) is an essential element for life and plays a crucial role in various biological processes. Fe acts as an electron transfer agent in many enzymes. For example, Fe is present in the heme group of hemoglobin and myoglobin, which are involved in oxygen transport in the body. Fe is also present in cytochromes, which are involved in electron transfer in the respiratory chain.
Function of Zn
Zinc (Zn) is also an essential element for life and plays a crucial role in various biological processes. Zn is involved in catalysis and structural stabilization. Zn is present in many enzymes, such as carbonic anhydrase, which catalyzes the reversible hydration of carbon dioxide. Zn is also present in many DNA and RNA binding proteins, where it stabilizes the structure of the molecule.
Conclusion
In conclusion, Fe and Zn are a pair of metal ions present in nature. Fe functions as an electron transfer agent, while Zn catalyzes hydrolysis reactions.
During oxygen transport by hemerythrin, oxygen is bound as:- a)O2– to one Fe(III) only
- b)HO2– to one Fe(III) only
- c)O22– to one Fe(II) and one Fe(III)
- d)O22– to two Fe(II)
Correct answer is option 'B'. Can you explain this answer?
During oxygen transport by hemerythrin, oxygen is bound as:
a)
O2– to one Fe(III) only
b)
HO2– to one Fe(III) only
c)
O22– to one Fe(II) and one Fe(III)
d)
O22– to two Fe(II)

|
Bijoy Patel answered |
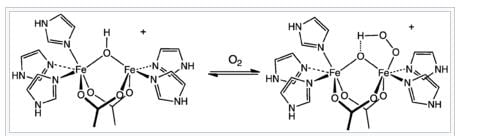
The mechanism of dioxygen binding is unusual. Most O2 carriers operate via formation of dioxygen complexes, but hemerythrin holds the O2 as a hydroperoxide (HO2, or -OOH−). The site that binds O2 consists of a pair of iron centres. The iron atoms are bound to the protein through the carboxylate side chains of a glutamate and aspartates as well as through five histidine residues. Hemerythrin and myohemerythrin are often described according to oxidation and ligation states of the iron center:
Fe2+—OH—Fe2+deoxy (reduced)
Fe2+—OH—Fe3+semi-met
Fe3+—O—Fe3+—OOH−oxy (oxidized)
Fe3+—OH—Fe3+— (any other ligand)met (oxidized)
The uptake of O2 by hemerythrin is accompanied by two-electron oxidation of the diferrous centre to produce a hydroperoxide (OOH−) complex. The binding of O2 is roughly described in this diagram:
In the transformation of oxyhaemoglobin to deoxyhaemoglobin:- a)Fe2+ in the low spin state changes to Fe2+ in the high spin state.
- b)Fe2+ in the low spin state changes to Fe3+ in the low spin state.
- c)Fe2+ in the high spin state changes to Fe2+ in the low spin state.
- d)Fe2+ in the high spin state changes to Fe3+ in the high spin state.
Correct answer is option 'A'. Can you explain this answer?
In the transformation of oxyhaemoglobin to deoxyhaemoglobin:
a)
Fe2+ in the low spin state changes to Fe2+ in the high spin state.
b)
Fe2+ in the low spin state changes to Fe3+ in the low spin state.
c)
Fe2+ in the high spin state changes to Fe2+ in the low spin state.
d)
Fe2+ in the high spin state changes to Fe3+ in the high spin state.

|
Anshul Mehra answered |
Understanding Oxyhaemoglobin and Deoxyhaemoglobin Transformation
The transformation of oxyhaemoglobin to deoxyhaemoglobin involves significant changes in the iron (Fe) state within the haem group.
Oxyhaemoglobin State
- In oxyhaemoglobin, the iron is in the Fe2+ state.
- This Fe2+ is typically in a low spin configuration due to the strong field ligands (oxygen) surrounding it.
Transition to Deoxyhaemoglobin
- Upon the release of oxygen (deoxygenation), the iron undergoes a transition.
- The Fe2+ in the low spin state changes to a high spin state.
Significance of High Spin State
- In the high spin state, the iron has unpaired electrons, which leads to a change in the geometry of the haem group.
- This conformational change is crucial as it allows haemoglobin to release oxygen more effectively.
Other Options Explained
- Option B is incorrect because Fe2+ does not change to Fe3+ in the low spin state; rather, it remains Fe2+.
- Option C is incorrect as it suggests a transition within the same oxidation state (Fe2+) without the influence of oxygen.
- Option D is also incorrect because it implies a change to Fe3+ while in the high spin state, which does not occur in this context.
Conclusion
The correct answer is option A because the transformation from oxyhaemoglobin to deoxyhaemoglobin indeed involves the change of Fe2+ from a low spin state to a high spin state, facilitating the release of oxygen.
The transformation of oxyhaemoglobin to deoxyhaemoglobin involves significant changes in the iron (Fe) state within the haem group.
Oxyhaemoglobin State
- In oxyhaemoglobin, the iron is in the Fe2+ state.
- This Fe2+ is typically in a low spin configuration due to the strong field ligands (oxygen) surrounding it.
Transition to Deoxyhaemoglobin
- Upon the release of oxygen (deoxygenation), the iron undergoes a transition.
- The Fe2+ in the low spin state changes to a high spin state.
Significance of High Spin State
- In the high spin state, the iron has unpaired electrons, which leads to a change in the geometry of the haem group.
- This conformational change is crucial as it allows haemoglobin to release oxygen more effectively.
Other Options Explained
- Option B is incorrect because Fe2+ does not change to Fe3+ in the low spin state; rather, it remains Fe2+.
- Option C is incorrect as it suggests a transition within the same oxidation state (Fe2+) without the influence of oxygen.
- Option D is also incorrect because it implies a change to Fe3+ while in the high spin state, which does not occur in this context.
Conclusion
The correct answer is option A because the transformation from oxyhaemoglobin to deoxyhaemoglobin indeed involves the change of Fe2+ from a low spin state to a high spin state, facilitating the release of oxygen.
Mg2+ is preferred in photosynthesis by chlorophyll because:- a)It has strong spin–orbit coupling.
- b)It has weak spin–orbit coupling.
- c)It is a heave metal.
- d)It binds strongly with chlorophyll.
Correct answer is option 'B'. Can you explain this answer?
Mg2+ is preferred in photosynthesis by chlorophyll because:
a)
It has strong spin–orbit coupling.
b)
It has weak spin–orbit coupling.
c)
It is a heave metal.
d)
It binds strongly with chlorophyll.

|
Niti Mukherjee answered |
Explanation:
Chlorophyll is a green pigment present in plants that is involved in photosynthesis, which is the process of converting light energy into chemical energy. Chlorophyll contains a central magnesium ion (Mg2+) which is essential for its function.
The reason why Mg2+ is preferred in photosynthesis by chlorophyll is because of its weak spin-orbit coupling. Spin-orbit coupling is the interaction between the spin and orbital motion of an electron. The strength of spin-orbit coupling is determined by the atomic number of the element. Elements with higher atomic numbers have stronger spin-orbit coupling.
Here's why Mg2+ is preferred in photosynthesis by chlorophyll:
1. Weak spin-orbit coupling: Mg2+ has a relatively low atomic number (12) compared to other metal ions, which means it has weaker spin-orbit coupling. This is beneficial for photosynthesis because it allows for efficient energy transfer between the chlorophyll molecules.
2. Essential for chlorophyll function: Mg2+ is an essential component of chlorophyll and is required for its function in photosynthesis. Without Mg2+, chlorophyll cannot absorb light and convert it into chemical energy.
3. Abundant in the environment: Mg2+ is abundant in the environment and is readily available for use by plants. This makes it a convenient choice for chlorophyll to use in photosynthesis.
In summary, Mg2+ is preferred in photosynthesis by chlorophyll because of its weak spin-orbit coupling, essential role in chlorophyll function, and abundance in the environment.
Chlorophyll is a green pigment present in plants that is involved in photosynthesis, which is the process of converting light energy into chemical energy. Chlorophyll contains a central magnesium ion (Mg2+) which is essential for its function.
The reason why Mg2+ is preferred in photosynthesis by chlorophyll is because of its weak spin-orbit coupling. Spin-orbit coupling is the interaction between the spin and orbital motion of an electron. The strength of spin-orbit coupling is determined by the atomic number of the element. Elements with higher atomic numbers have stronger spin-orbit coupling.
Here's why Mg2+ is preferred in photosynthesis by chlorophyll:
1. Weak spin-orbit coupling: Mg2+ has a relatively low atomic number (12) compared to other metal ions, which means it has weaker spin-orbit coupling. This is beneficial for photosynthesis because it allows for efficient energy transfer between the chlorophyll molecules.
2. Essential for chlorophyll function: Mg2+ is an essential component of chlorophyll and is required for its function in photosynthesis. Without Mg2+, chlorophyll cannot absorb light and convert it into chemical energy.
3. Abundant in the environment: Mg2+ is abundant in the environment and is readily available for use by plants. This makes it a convenient choice for chlorophyll to use in photosynthesis.
In summary, Mg2+ is preferred in photosynthesis by chlorophyll because of its weak spin-orbit coupling, essential role in chlorophyll function, and abundance in the environment.
Molybdoenzymes can both oxidize as well as reduce the substrates, because:- a)Mo(VI) is more stable than Mo(IV).
- b)Mo(IV) can transfer oxygen atom to the substrate and Mo(VI) can abstract oxygen atom from the substrate.
- c)Conversion of Mo(VI) to Mo(IV) is not favoured.
- d)Mo(VI) can transfer oxygen atom to the substrate and Mo(IV) can abstract oxygen atom from the substrate.
Correct answer is option 'D'. Can you explain this answer?
Molybdoenzymes can both oxidize as well as reduce the substrates, because:
a)
Mo(VI) is more stable than Mo(IV).
b)
Mo(IV) can transfer oxygen atom to the substrate and Mo(VI) can abstract oxygen atom from the substrate.
c)
Conversion of Mo(VI) to Mo(IV) is not favoured.
d)
Mo(VI) can transfer oxygen atom to the substrate and Mo(IV) can abstract oxygen atom from the substrate.
|
|
Pooja Choudhury answered |
-Molybdoenzymes are an ancient protein family found in phylogenetically and ecologically diverse prokaryotes. Under anaerobic conditions, respiratory molybdoenzymes catalyze redox reactions that transfer electrons to a variety of substrates that act as terminal electron acceptors for energy generation.
-Molybdoenzymes can both oxidize as well as reduce the substrates, because Mo(VI) can transfer oxygen atom to the substrate and Mo(IV) can abstract oxygen atom from the substrate.In biological systems, the metal ion involved in the dioxygen transport besides Fe is:- a)Co
- b)Zn
- c)Mg
- d)Cu
Correct answer is option 'D'. Can you explain this answer?
In biological systems, the metal ion involved in the dioxygen transport besides Fe is:
a)
Co
b)
Zn
c)
Mg
d)
Cu

|
Rishabh Mehta answered |
Metal Ion Involved in Dioxygen Transport Besides Fe
Dioxygen transport is an essential process in biological systems that involves metal ions. Apart from iron (Fe), copper (Cu) is also involved in the dioxygen transport process.
Copper in Dioxygen Transport
Copper is involved in the transport of dioxygen in hemocyanin and hemerythrin proteins. These proteins are present in invertebrates, such as arthropods and mollusks, and function similarly to hemoglobin in vertebrates.
Hemocyanin
Hemocyanin is a copper-containing protein that is found in the blood of arthropods and mollusks. It binds to and transports dioxygen to the tissues. Hemocyanin has a blue color due to the presence of copper ions, which are coordinated by histidine residues in the protein.
Hemerythrin
Hemerythrin is another dioxygen transport protein that contains copper ions. It is found in the blood of some marine invertebrates, such as brachiopods and sipunculids. Hemerythrin binds to dioxygen reversibly and transports it to the tissues.
Conclusion
In conclusion, besides iron, copper is also involved in the dioxygen transport process in biological systems. Copper-containing proteins, such as hemocyanin and hemerythrin, function similarly to hemoglobin in vertebrates and transport dioxygen to the tissues.
Dioxygen transport is an essential process in biological systems that involves metal ions. Apart from iron (Fe), copper (Cu) is also involved in the dioxygen transport process.
Copper in Dioxygen Transport
Copper is involved in the transport of dioxygen in hemocyanin and hemerythrin proteins. These proteins are present in invertebrates, such as arthropods and mollusks, and function similarly to hemoglobin in vertebrates.
Hemocyanin
Hemocyanin is a copper-containing protein that is found in the blood of arthropods and mollusks. It binds to and transports dioxygen to the tissues. Hemocyanin has a blue color due to the presence of copper ions, which are coordinated by histidine residues in the protein.
Hemerythrin
Hemerythrin is another dioxygen transport protein that contains copper ions. It is found in the blood of some marine invertebrates, such as brachiopods and sipunculids. Hemerythrin binds to dioxygen reversibly and transports it to the tissues.
Conclusion
In conclusion, besides iron, copper is also involved in the dioxygen transport process in biological systems. Copper-containing proteins, such as hemocyanin and hemerythrin, function similarly to hemoglobin in vertebrates and transport dioxygen to the tissues.
The amino acid side chain high affinity for Ca2+ and Cu2+ in metallo–proteins is:- a)Carboxylate in both the cases.
- b)Imidazole in both the cases.
- c)Carboxylate for Ca2+ and imidazo le for Cu2+.
- d)Imidazole for Ca2+ and carboxylate for Cu2+.
Correct answer is option 'C'. Can you explain this answer?
The amino acid side chain high affinity for Ca2+ and Cu2+ in metallo–proteins is:
a)
Carboxylate in both the cases.
b)
Imidazole in both the cases.
c)
Carboxylate for Ca2+ and imidazo le for Cu2+.
d)
Imidazole for Ca2+ and carboxylate for Cu2+.

|
Ipsita Chopra answered |
Metalloproteins are proteins that contain metal ions as an integral part of their structure and function. The amino acid side chains in these proteins have a high affinity for metal ions such as Ca2+ and Cu2+. The correct answer is option C, which states that carboxylate is the high-affinity side chain for Ca2+ and imidazole is the high-affinity side chain for Cu2+.
Carboxylate Side Chain for Ca2+
The carboxylate side chain is a negatively charged group that is present in amino acids such as aspartate and glutamate. This side chain has a high affinity for metal ions such as Ca2+ due to its negative charge. Ca2+ ions are essential for many biological processes such as muscle contraction, neurotransmitter release, and blood clotting. Metalloproteins that bind Ca2+ ions include calmodulin, troponin C, and osteocalcin.
Imidazole Side Chain for Cu2+
The imidazole side chain is a nitrogen-containing group that is present in the amino acid histidine. This side chain has a high affinity for metal ions such as Cu2+ due to its ability to coordinate with the metal ion through its nitrogen atoms. Cu2+ ions are essential for many biological processes such as electron transport, oxidative metabolism, and iron absorption. Metalloproteins that bind Cu2+ ions include ceruloplasmin, cytochrome c oxidase, and dopamine β-hydroxylase.
In conclusion, the amino acid side chains in metalloproteins have a high affinity for metal ions such as Ca2+ and Cu2+. The carboxylate side chain is the high-affinity side chain for Ca2+ ions, while the imidazole side chain is the high-affinity side chain for Cu2+ ions.
Carboxylate Side Chain for Ca2+
The carboxylate side chain is a negatively charged group that is present in amino acids such as aspartate and glutamate. This side chain has a high affinity for metal ions such as Ca2+ due to its negative charge. Ca2+ ions are essential for many biological processes such as muscle contraction, neurotransmitter release, and blood clotting. Metalloproteins that bind Ca2+ ions include calmodulin, troponin C, and osteocalcin.
Imidazole Side Chain for Cu2+
The imidazole side chain is a nitrogen-containing group that is present in the amino acid histidine. This side chain has a high affinity for metal ions such as Cu2+ due to its ability to coordinate with the metal ion through its nitrogen atoms. Cu2+ ions are essential for many biological processes such as electron transport, oxidative metabolism, and iron absorption. Metalloproteins that bind Cu2+ ions include ceruloplasmin, cytochrome c oxidase, and dopamine β-hydroxylase.
In conclusion, the amino acid side chains in metalloproteins have a high affinity for metal ions such as Ca2+ and Cu2+. The carboxylate side chain is the high-affinity side chain for Ca2+ ions, while the imidazole side chain is the high-affinity side chain for Cu2+ ions.
Oxidized form of enzyme Catalase (structure A); prepared by the reaction of [Fe(P)]+ (P = porphyrin) with H2O2 has a green color because of: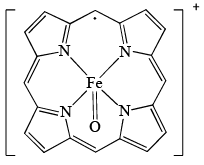 A (substituents on ring are removed for clarity)
A (substituents on ring are removed for clarity)- a)Oxidat ion state of iron changed fro m FeIII to FeIV.
- b)Porphyrin ring is oxidized by one electron.
- c)π–π* transit ion appears in the visible region.
- d)FeIV is coordinated with anionic tyrosinnate ligand in axial position.
Correct answer is option 'A'. Can you explain this answer?
Oxidized form of enzyme Catalase (structure A); prepared by the reaction of [Fe(P)]+ (P = porphyrin) with H2O2 has a green color because of:
A (substituents on ring are removed for clarity)
a)
Oxidat ion state of iron changed fro m FeIII to FeIV.
b)
Porphyrin ring is oxidized by one electron.
c)
π–π* transit ion appears in the visible region.
d)
FeIV is coordinated with anionic tyrosinnate ligand in axial position.

|
Mullapudi Divyasree answered |
Basically, depending on oxidation state of the metal ion, it will have different absorptions in uv to visible spectrum.
Therefore, H2O2 is an oxidising agent which changes the oxidation state of Fe3+ to Fe4+
Therefore, H2O2 is an oxidising agent which changes the oxidation state of Fe3+ to Fe4+
Zn in carbonic anhydrase is coordinated by three hist idine and one water molecule. The reaction of CO2 with this enzyme is an example of:- a)Electrophilic addition.
- b)Electron transfer.
- c)Nucleophilic addit io n.
- d)Electrophilic subst itution.
Correct answer is option 'C'. Can you explain this answer?
Zn in carbonic anhydrase is coordinated by three hist idine and one water molecule. The reaction of CO2 with this enzyme is an example of:
a)
Electrophilic addition.
b)
Electron transfer.
c)
Nucleophilic addit io n.
d)
Electrophilic subst itution.
|
|
Pooja Choudhury answered |
CO2 acts as an electrophile and H2O as a nucleophile which attacks CO2. Hence, electrophilic addition takes place.
Which of the following metals is present at the active site of carbonic anhydrase enzyme?- a)Iron (Fe)
- b)Zinc (Zn)
- c)Copper (Cu)
- d)e)b) Zinc (Zn)c) Copper (Cu)
Correct answer is option 'B'. Can you explain this answer?
Which of the following metals is present at the active site of carbonic anhydrase enzyme?
a)
Iron (Fe)
b)
Zinc (Zn)
c)
Copper (Cu)
d)
e)b) Zinc (Zn)c) Copper (Cu)

|
Ishani Dasgupta answered |
Active Site of Carbonic Anhydrase
Carbonic anhydrase is a crucial enzyme that catalyzes the reversible reaction between carbon dioxide and water to form bicarbonate and protons. The presence of a metal ion at its active site is essential for its enzymatic function.
Metal Ion in Carbonic Anhydrase
- The active site of carbonic anhydrase contains Zinc (Zn).
- Zinc plays a vital role in facilitating the reaction by stabilizing the negative charge that develops during the conversion of carbon dioxide and water.
Function of Zinc in the Enzyme
- Catalytic Role: Zinc has a strong affinity for water molecules, which helps in the conversion of carbon dioxide into bicarbonate.
- Hydration: The presence of zinc allows water molecules to be more nucleophilic, enhancing the reaction rate.
Comparison with Other Metals
- Iron (Fe): While iron is important in many biological processes, it is not involved in carbonic anhydrase activity.
- Copper (Cu): Copper also has enzymatic roles, but it does not participate in the active site of carbonic anhydrase.
Conclusion
In summary, the correct answer is Zinc (Zn) due to its critical role at the active site of carbonic anhydrase, enabling efficient catalysis of the carbon dioxide hydration reaction. This unique feature highlights the importance of metal ions in enzymatic functions.
Carbonic anhydrase is a crucial enzyme that catalyzes the reversible reaction between carbon dioxide and water to form bicarbonate and protons. The presence of a metal ion at its active site is essential for its enzymatic function.
Metal Ion in Carbonic Anhydrase
- The active site of carbonic anhydrase contains Zinc (Zn).
- Zinc plays a vital role in facilitating the reaction by stabilizing the negative charge that develops during the conversion of carbon dioxide and water.
Function of Zinc in the Enzyme
- Catalytic Role: Zinc has a strong affinity for water molecules, which helps in the conversion of carbon dioxide into bicarbonate.
- Hydration: The presence of zinc allows water molecules to be more nucleophilic, enhancing the reaction rate.
Comparison with Other Metals
- Iron (Fe): While iron is important in many biological processes, it is not involved in carbonic anhydrase activity.
- Copper (Cu): Copper also has enzymatic roles, but it does not participate in the active site of carbonic anhydrase.
Conclusion
In summary, the correct answer is Zinc (Zn) due to its critical role at the active site of carbonic anhydrase, enabling efficient catalysis of the carbon dioxide hydration reaction. This unique feature highlights the importance of metal ions in enzymatic functions.
The bio logical functions of the cytochrome P450 and myoglobin are, respectively:- a)Oxidation of alkene and O2 storage
- b)O2 transport and O2 storage
- c)O2 storage and electron carrier
- d)Electron carrier and O2 transport
Correct answer is option 'A'. Can you explain this answer?
The bio logical functions of the cytochrome P450 and myoglobin are, respectively:
a)
Oxidation of alkene and O2 storage
b)
O2 transport and O2 storage
c)
O2 storage and electron carrier
d)
Electron carrier and O2 transport

|
Baishali Bajaj answered |
Cytochrome P450 enzymes are essential for the metabolism of many medications. ... Knowledge of the most important drugs metabolized by cytochrome P450 enzymes, as well as the most potent inhibiting and inducing drugs, can help minimize the possibility of adverse drug reactions and interactions.
Identify the function of hemocyanin and the metal responsible for it:- a)O2 transport and Fe
- b)O2 transport and Cu
- c)Electron transport and Fe
- d)Electron transport and Cu
Correct answer is option 'B'. Can you explain this answer?
Identify the function of hemocyanin and the metal responsible for it:
a)
O2 transport and Fe
b)
O2 transport and Cu
c)
Electron transport and Fe
d)
Electron transport and Cu

|
Palak Singh answered |
Function and Metal Responsible for Hemocyanin
Function:
Hemocyanin is a copper-containing protein that is responsible for transporting oxygen in the blood of various invertebrates, such as crustaceans, mollusks, and arachnids. It is an alternative to hemoglobin, which is the oxygen-transporting protein found in vertebrates.
Metal Responsible:
The metal responsible for the function of hemocyanin is copper. Copper is a transition metal that has the ability to bind to oxygen molecules. In hemocyanin, copper ions bind to oxygen molecules, allowing for the transport of oxygen in the blood.
Hemocyanin and Oxygen Transport:
Hemocyanin is able to transport oxygen due to its unique structure. It consists of multiple subunits, each containing a copper ion that can bind to an oxygen molecule. When oxygen is present, the copper ions in hemocyanin become oxidized, causing a color change from blue to colorless. This allows for the release of oxygen to the tissues that need it.
Hemocyanin has a higher affinity for oxygen than hemoglobin, which allows for efficient oxygen transport even in low oxygen environments. This is particularly important for invertebrates that live in aquatic environments with low oxygen levels.
Conclusion:
In summary, hemocyanin is a copper-containing protein that is responsible for transporting oxygen in the blood of invertebrates. Copper is the metal responsible for the function of hemocyanin, as it is able to bind to oxygen molecules. Hemocyanin's unique structure allows for efficient oxygen transport, even in low oxygen environments.
Function:
Hemocyanin is a copper-containing protein that is responsible for transporting oxygen in the blood of various invertebrates, such as crustaceans, mollusks, and arachnids. It is an alternative to hemoglobin, which is the oxygen-transporting protein found in vertebrates.
Metal Responsible:
The metal responsible for the function of hemocyanin is copper. Copper is a transition metal that has the ability to bind to oxygen molecules. In hemocyanin, copper ions bind to oxygen molecules, allowing for the transport of oxygen in the blood.
Hemocyanin and Oxygen Transport:
Hemocyanin is able to transport oxygen due to its unique structure. It consists of multiple subunits, each containing a copper ion that can bind to an oxygen molecule. When oxygen is present, the copper ions in hemocyanin become oxidized, causing a color change from blue to colorless. This allows for the release of oxygen to the tissues that need it.
Hemocyanin has a higher affinity for oxygen than hemoglobin, which allows for efficient oxygen transport even in low oxygen environments. This is particularly important for invertebrates that live in aquatic environments with low oxygen levels.
Conclusion:
In summary, hemocyanin is a copper-containing protein that is responsible for transporting oxygen in the blood of invertebrates. Copper is the metal responsible for the function of hemocyanin, as it is able to bind to oxygen molecules. Hemocyanin's unique structure allows for efficient oxygen transport, even in low oxygen environments.
The Fe—Npprphyrin bond distances in the deoxy and oxy-hemoglobin, respect ively are:- a)~2.1 and 2.0 Å
- b)~2.0 and 2.0 Å
- c)~2.2 and 2.3 Å
- d)~2.3 and 2.5 Å
Correct answer is option 'A'. Can you explain this answer?
The Fe—Npprphyrin bond distances in the deoxy and oxy-hemoglobin, respect ively are:
a)
~2.1 and 2.0 Å
b)
~2.0 and 2.0 Å
c)
~2.2 and 2.3 Å
d)
~2.3 and 2.5 Å

|
Partho Gupta answered |
Festival of Lights, also known as Diwali, is a Hindu festival celebrated every year in late October or early November. The festival is a celebration of the victory of light over darkness, good over evil, and knowledge over ignorance. During the festival, people decorate their homes with lights, lamps, and rangolis. They offer prayers to the goddess Lakshmi and Lord Ganesha for wealth and prosperity. People also exchange gifts and sweets with family, friends, and neighbors. The festival usually lasts for five days, with the third day being the main day of celebration.
Fe2+ -porphyrins fail to exhibit reversible oxygen transport and cannot differentiate CO from O2. However, the hemoglobin is free from both these pit falls. Among the following the correct set of statements is:(A) Fe2+-porphyrins undergo μ-oxodimer formation and the same is prevented in case of the hemoglobin.
(B) Fe–CO bond strength is much low in case of hemoglobin when compared to the Fe2+ -porthyrins.
(C) While Fe–CO is linear, Fe–O2 is bent and is recognized by hemoglobin.
(D) The interlinked four monomeric units in the hemoglobin are responsible to overcome the pitfalls. - a)A and B
- b)A and C
- c)C and D
- d)B and D
Correct answer is option 'B'. Can you explain this answer?
Fe2+ -porphyrins fail to exhibit reversible oxygen transport and cannot differentiate CO from O2. However, the hemoglobin is free from both these pit falls. Among the following the correct set of statements is:
(A) Fe2+-porphyrins undergo μ-oxodimer formation and the same is prevented in case of the hemoglobin.
(B) Fe–CO bond strength is much low in case of hemoglobin when compared to the Fe2+ -porthyrins.
(C) While Fe–CO is linear, Fe–O2 is bent and is recognized by hemoglobin.
(D) The interlinked four monomeric units in the hemoglobin are responsible to overcome the pitfalls.
(B) Fe–CO bond strength is much low in case of hemoglobin when compared to the Fe2+ -porthyrins.
(C) While Fe–CO is linear, Fe–O2 is bent and is recognized by hemoglobin.
(D) The interlinked four monomeric units in the hemoglobin are responsible to overcome the pitfalls.
a)
A and B
b)
A and C
c)
C and D
d)
B and D

|
Anshika Chavan answered |
Explanation:
Fe2-porphyrins and Hemoglobin:
- Fe2-porphyrins and hemoglobin both contain a heme group which consists of an iron atom bound to a porphyrin ring.
- Fe2-porphyrins are synthetic compounds while hemoglobin is a natural protein found in red blood cells.
- Fe2-porphyrins cannot differentiate between CO and O2 due to the lack of a specific binding site while hemoglobin can distinguish between the two gases.
- Fe2-porphyrins do not exhibit reversible oxygen transport, while hemoglobin can transport oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs.
Bond Strength:
- The bond strength between Fe and CO is much lower in hemoglobin than in Fe2-porphyrins. This is due to the unique environment created by the protein structure of hemoglobin which weakens the bond between Fe and CO.
FeCO and FeO2:
- FeCO is a linear molecule while FeO2 is bent. This difference in geometry is due to the presence of two lone pairs on the oxygen atoms in FeO2.
- Hemoglobin recognizes the bent geometry of FeO2 and binds to it specifically, allowing for the transport of oxygen in the body.
Interlinked Monomeric Units:
- The interlinked four monomeric units in hemoglobin work together to overcome the pitfalls of Fe2-porphyrins.
- The protein structure of hemoglobin creates a specific binding site for oxygen, allowing for efficient oxygen transport in the body.
- The interlinked monomeric units also allow for the cooperative binding of oxygen, meaning that each oxygen molecule that binds to hemoglobin increases the affinity of the remaining sites for oxygen.
Therefore, option B is the correct answer as it correctly identifies the difference in bond strength between FeCO in hemoglobin and Fe2-porphyrins.
Fe2-porphyrins and Hemoglobin:
- Fe2-porphyrins and hemoglobin both contain a heme group which consists of an iron atom bound to a porphyrin ring.
- Fe2-porphyrins are synthetic compounds while hemoglobin is a natural protein found in red blood cells.
- Fe2-porphyrins cannot differentiate between CO and O2 due to the lack of a specific binding site while hemoglobin can distinguish between the two gases.
- Fe2-porphyrins do not exhibit reversible oxygen transport, while hemoglobin can transport oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs.
Bond Strength:
- The bond strength between Fe and CO is much lower in hemoglobin than in Fe2-porphyrins. This is due to the unique environment created by the protein structure of hemoglobin which weakens the bond between Fe and CO.
FeCO and FeO2:
- FeCO is a linear molecule while FeO2 is bent. This difference in geometry is due to the presence of two lone pairs on the oxygen atoms in FeO2.
- Hemoglobin recognizes the bent geometry of FeO2 and binds to it specifically, allowing for the transport of oxygen in the body.
Interlinked Monomeric Units:
- The interlinked four monomeric units in hemoglobin work together to overcome the pitfalls of Fe2-porphyrins.
- The protein structure of hemoglobin creates a specific binding site for oxygen, allowing for efficient oxygen transport in the body.
- The interlinked monomeric units also allow for the cooperative binding of oxygen, meaning that each oxygen molecule that binds to hemoglobin increases the affinity of the remaining sites for oxygen.
Therefore, option B is the correct answer as it correctly identifies the difference in bond strength between FeCO in hemoglobin and Fe2-porphyrins.
At pH 7.2 and 10 Torr oxygen partial pressure, the extent of O2 binding is:- a)High for both hemoglobin and myoglobin
- b)High for hemoglobin and low for myoglobin
- c)High for myoglobin and low for hemoglobin
- d)Low for both hemoglobin and myoglobin
Correct answer is option 'C'. Can you explain this answer?
At pH 7.2 and 10 Torr oxygen partial pressure, the extent of O2 binding is:
a)
High for both hemoglobin and myoglobin
b)
High for hemoglobin and low for myoglobin
c)
High for myoglobin and low for hemoglobin
d)
Low for both hemoglobin and myoglobin

|
Aryan Choudhary answered |
PH and Oxygen Partial Pressure and their effect on O2 binding
pH and oxygen partial pressure are two important factors that affect the binding of oxygen to hemoglobin and myoglobin. Hemoglobin and myoglobin are both proteins involved in oxygen transport and storage in the body.
pH: pH is the measure of acidity or basicity of a solution. The pH of blood is tightly regulated within a narrow range of 7.35-7.45. Any change in pH can affect the binding of oxygen to hemoglobin and myoglobin.
Oxygen Partial Pressure: Oxygen partial pressure is the measure of the concentration of oxygen in a mixture of gases. It is expressed in Torr or mmHg. The oxygen partial pressure in arterial blood is around 100 Torr.
Extent of O2 Binding at pH 7.2 and 10 Torr oxygen partial pressure
At pH 7.2 and 10 Torr oxygen partial pressure, the extent of O2 binding is high for myoglobin and low for hemoglobin. This is because:
- Myoglobin has a higher affinity for oxygen than hemoglobin at low oxygen partial pressures. This means that myoglobin can bind oxygen more easily than hemoglobin at low oxygen concentrations.
- At pH 7.2, the affinity of myoglobin for oxygen is further increased due to the Bohr effect. The Bohr effect is a phenomenon where the binding of oxygen to hemoglobin and myoglobin is influenced by the pH of the blood. At low pH, the affinity of myoglobin for oxygen is increased due to the release of protons, which stabilizes the T state of myoglobin and facilitates oxygen binding.
- Hemoglobin, on the other hand, has a lower affinity for oxygen at low oxygen partial pressures. This is because hemoglobin undergoes a conformational change from the T state to the R state upon binding to oxygen. This conformational change is facilitated by the release of protons, which is why hemoglobin has a higher affinity for oxygen at high pH.
Therefore, at pH 7.2 and 10 Torr oxygen partial pressure, myoglobin binds oxygen more easily than hemoglobin.
pH and oxygen partial pressure are two important factors that affect the binding of oxygen to hemoglobin and myoglobin. Hemoglobin and myoglobin are both proteins involved in oxygen transport and storage in the body.
pH: pH is the measure of acidity or basicity of a solution. The pH of blood is tightly regulated within a narrow range of 7.35-7.45. Any change in pH can affect the binding of oxygen to hemoglobin and myoglobin.
Oxygen Partial Pressure: Oxygen partial pressure is the measure of the concentration of oxygen in a mixture of gases. It is expressed in Torr or mmHg. The oxygen partial pressure in arterial blood is around 100 Torr.
Extent of O2 Binding at pH 7.2 and 10 Torr oxygen partial pressure
At pH 7.2 and 10 Torr oxygen partial pressure, the extent of O2 binding is high for myoglobin and low for hemoglobin. This is because:
- Myoglobin has a higher affinity for oxygen than hemoglobin at low oxygen partial pressures. This means that myoglobin can bind oxygen more easily than hemoglobin at low oxygen concentrations.
- At pH 7.2, the affinity of myoglobin for oxygen is further increased due to the Bohr effect. The Bohr effect is a phenomenon where the binding of oxygen to hemoglobin and myoglobin is influenced by the pH of the blood. At low pH, the affinity of myoglobin for oxygen is increased due to the release of protons, which stabilizes the T state of myoglobin and facilitates oxygen binding.
- Hemoglobin, on the other hand, has a lower affinity for oxygen at low oxygen partial pressures. This is because hemoglobin undergoes a conformational change from the T state to the R state upon binding to oxygen. This conformational change is facilitated by the release of protons, which is why hemoglobin has a higher affinity for oxygen at high pH.
Therefore, at pH 7.2 and 10 Torr oxygen partial pressure, myoglobin binds oxygen more easily than hemoglobin.
In metal ion catalysis, what is the primary function of the metal ion?- a)To provide electrons for the reaction
- b)To stabilize negative charges on reaction intermediates
- c)To act as a substrate for the reaction
- d)To increase the temperature of the reaction
Correct answer is option 'B'. Can you explain this answer?
In metal ion catalysis, what is the primary function of the metal ion?
a)
To provide electrons for the reaction
b)
To stabilize negative charges on reaction intermediates
c)
To act as a substrate for the reaction
d)
To increase the temperature of the reaction

|
Asf Institute answered |
To stabilize negative charges on reaction intermediates
The primary function of the metal ion in metal ion catalysis is to stabilize negative charges on reaction intermediates.
Key Points
- Stabilization of negative charges:
- Metal ions in catalytic processes often stabilize negatively charged intermediates, which can be crucial for the reaction to proceed.
- This stabilization lowers the activation energy, making the reaction more feasible.
- Coordination chemistry:
- Metal ions can coordinate with substrates to form more reactive complexes, facilitating the reaction.
The metal present at the active site of the protein Carboxypept idase A is:- a)Zinc
- b)Molybdenum
- c)Magnesium
- d)Cobalt
Correct answer is option 'A'. Can you explain this answer?
The metal present at the active site of the protein Carboxypept idase A is:
a)
Zinc
b)
Molybdenum
c)
Magnesium
d)
Cobalt

|
Anagha Bajaj answered |
The metal present at the active site of the protein Carboxypeptidase A is Zinc.
Carboxypeptidases are enzymes that hydrolyze peptide bonds at the C-terminus (carboxyl end) of proteins and peptides. Carboxypeptidase A is one of the major forms of carboxypeptidases found in mammals and is involved in the digestion and processing of proteins.
Importance of Metal Ions in Enzyme Catalysis:
Metal ions play a critical role in the catalytic function of many enzymes. They can function as cofactors, which are essential for the enzyme's activity, or as activators/modulators, which enhance or modulate the enzyme's activity. In the case of carboxypeptidase A, the metal ion present at the active site acts as a cofactor, facilitating the enzyme's catalytic activity.
Role of Zinc in Carboxypeptidase A:
Zinc is the metal ion present at the active site of Carboxypeptidase A. It plays a crucial role in the enzyme's catalytic mechanism. The zinc ion coordinates with specific amino acid residues in the active site of the enzyme, forming a stable complex.
Coordination of Zinc Ion:
The zinc ion in carboxypeptidase A is coordinated by three histidine residues (His69, His196, and His69) and a water molecule. These residues, along with the zinc ion, form a tetrahedral coordination complex.
Stabilization of Transition State:
The presence of the zinc ion in the active site of carboxypeptidase A helps in the stabilization of the transition state during catalysis. The zinc ion can polarize the peptide bond, facilitating its hydrolysis. It also helps in the orientation and positioning of the substrate, enabling efficient catalysis.
General Function of Metal Ions in Enzymes:
Metal ions in enzymes can participate in various catalytic mechanisms, such as acid-base catalysis, redox reactions, and stabilization of reaction intermediates. They can also enhance the binding of substrates to the active site and promote conformational changes required for catalysis.
In summary, the metal ion present at the active site of Carboxypeptidase A is Zinc. It acts as a cofactor, facilitating the enzyme's catalytic activity by coordinating with specific amino acid residues and stabilizing the transition state during peptide bond hydrolysis.
Carboxypeptidases are enzymes that hydrolyze peptide bonds at the C-terminus (carboxyl end) of proteins and peptides. Carboxypeptidase A is one of the major forms of carboxypeptidases found in mammals and is involved in the digestion and processing of proteins.
Importance of Metal Ions in Enzyme Catalysis:
Metal ions play a critical role in the catalytic function of many enzymes. They can function as cofactors, which are essential for the enzyme's activity, or as activators/modulators, which enhance or modulate the enzyme's activity. In the case of carboxypeptidase A, the metal ion present at the active site acts as a cofactor, facilitating the enzyme's catalytic activity.
Role of Zinc in Carboxypeptidase A:
Zinc is the metal ion present at the active site of Carboxypeptidase A. It plays a crucial role in the enzyme's catalytic mechanism. The zinc ion coordinates with specific amino acid residues in the active site of the enzyme, forming a stable complex.
Coordination of Zinc Ion:
The zinc ion in carboxypeptidase A is coordinated by three histidine residues (His69, His196, and His69) and a water molecule. These residues, along with the zinc ion, form a tetrahedral coordination complex.
Stabilization of Transition State:
The presence of the zinc ion in the active site of carboxypeptidase A helps in the stabilization of the transition state during catalysis. The zinc ion can polarize the peptide bond, facilitating its hydrolysis. It also helps in the orientation and positioning of the substrate, enabling efficient catalysis.
General Function of Metal Ions in Enzymes:
Metal ions in enzymes can participate in various catalytic mechanisms, such as acid-base catalysis, redox reactions, and stabilization of reaction intermediates. They can also enhance the binding of substrates to the active site and promote conformational changes required for catalysis.
In summary, the metal ion present at the active site of Carboxypeptidase A is Zinc. It acts as a cofactor, facilitating the enzyme's catalytic activity by coordinating with specific amino acid residues and stabilizing the transition state during peptide bond hydrolysis.
When a reduced cytochrome transfers an electron from its Fe(II) to the bound O2:- a)The bond order or O2 is reduced by one and ν02 decreases.
- b)A metal bound superoxide is formed and ν02 decrease.
- c)A metal bound superoxide is formed and ν02 increase.
- d)The bond order or O2 is reduced by one and ν02 increases.
Correct answer is option 'B'. Can you explain this answer?
When a reduced cytochrome transfers an electron from its Fe(II) to the bound O2:
a)
The bond order or O2 is reduced by one and ν02 decreases.
b)
A metal bound superoxide is formed and ν02 decrease.
c)
A metal bound superoxide is formed and ν02 increase.
d)
The bond order or O2 is reduced by one and ν02 increases.

|
Madhavan Iyer answered |
Explanation:
When a reduced cytochrome transfers an electron from its Fe(II) to the bound O2, a metal-bound superoxide is formed and O2 decreases. This can be explained as follows:
Formation of Metal-Bound Superoxide:
When a reduced cytochrome transfers an electron to the bound O2, the O2 molecule becomes superoxide (O2-), which is bound to the metal ion (Fe(III)) in the cytochrome. The Fe(III)-superoxide complex is stabilized by coordination of the superoxide to the metal ion through one of its oxygen atoms. This complex is a powerful oxidizing agent and can react with other molecules, such as proteins and lipids, causing damage to cells.
Decrease in O2:
When the electron is transferred from the cytochrome to the O2 molecule, the bond order of O2 is reduced by one, which means that the O-O bond is weakened. This weakens the O2 molecule, making it more reactive and prone to react with other molecules. As a result, the O2 molecule decreases in concentration.
Conclusion:
Thus, when a reduced cytochrome transfers an electron from its Fe(II) to the bound O2, a metal-bound superoxide is formed, and O2 decreases in concentration.
When a reduced cytochrome transfers an electron from its Fe(II) to the bound O2, a metal-bound superoxide is formed and O2 decreases. This can be explained as follows:
Formation of Metal-Bound Superoxide:
When a reduced cytochrome transfers an electron to the bound O2, the O2 molecule becomes superoxide (O2-), which is bound to the metal ion (Fe(III)) in the cytochrome. The Fe(III)-superoxide complex is stabilized by coordination of the superoxide to the metal ion through one of its oxygen atoms. This complex is a powerful oxidizing agent and can react with other molecules, such as proteins and lipids, causing damage to cells.
Decrease in O2:
When the electron is transferred from the cytochrome to the O2 molecule, the bond order of O2 is reduced by one, which means that the O-O bond is weakened. This weakens the O2 molecule, making it more reactive and prone to react with other molecules. As a result, the O2 molecule decreases in concentration.
Conclusion:
Thus, when a reduced cytochrome transfers an electron from its Fe(II) to the bound O2, a metal-bound superoxide is formed, and O2 decreases in concentration.
The number of histidine amino acid nitrogen atoms coordinated to bimetallic active site of oxyhemocyanin and oxyhemerythrine, respectively, are:- a)2, 3 and 3, 3
- b)3, 3 and 2, 3
- c)3, 3 and 2, 2
- d)2, 4 and 3, 2
Correct answer is option 'B'. Can you explain this answer?
The number of histidine amino acid nitrogen atoms coordinated to bimetallic active site of oxyhemocyanin and oxyhemerythrine, respectively, are:
a)
2, 3 and 3, 3
b)
3, 3 and 2, 3
c)
3, 3 and 2, 2
d)
2, 4 and 3, 2

|
Edurev.iitjam answered |
- The number of histidine amino acid nitrogen atoms coordinated to the bimetallic active site of oxyhemocyanin and oxyhemerythrin, respectively, are determined as follows:
- Oxyhemocyanin:
- Contains a dicopper (Cu22) active site.
- Each copper ion is coordinated by three histidine nitrogen atoms.
- Total for the bimetallic site: 3 histidine N per Cu × 2 Cu = 3, 3.
-
- Oxyhemerythrin:
- Contains a diiron (Fe22) active site.
- One iron is coordinated by three histidine nitrogen atoms, and the other by two histidine nitrogen atoms.
- Total for the bimetallic site: 3 histidine N (Fe11) + 2 histidine N (Fe22) = 2, 3.
-
Final Answer:
b) 3, 3 and 2, 3
Carboxypeptidase contains:- a)Zn (II) and hydrolyses CO2.
- b)Mg (II) and hydro lyses CO2.
- c)Zn (II) and hydrolyses peptide bonds.
- d)Mg (II) and hydrolyses peptide bonds.
Correct answer is option 'C'. Can you explain this answer?
Carboxypeptidase contains:
a)
Zn (II) and hydrolyses CO2.
b)
Mg (II) and hydro lyses CO2.
c)
Zn (II) and hydrolyses peptide bonds.
d)
Mg (II) and hydrolyses peptide bonds.

|
Sarthak Chavan answered |
Carboxypeptidase is an enzyme that hydrolyzes peptide bonds in proteins and peptides. It is found in the pancreatic juice and intestinal mucosa of animals. The enzyme is classified as a hydrolase, which means it breaks down molecules by adding water. Carboxypeptidase contains a zinc ion (Zn (II)) in its active site, which is essential for its catalytic function.
The mechanism of action of carboxypeptidase involves the binding of the substrate (a peptide or protein molecule) to the active site of the enzyme. The zinc ion acts as a Lewis acid, which means it accepts a pair of electrons from the peptide bond, thereby weakening the bond. This makes it easier for a water molecule to attack the peptide bond and break it, resulting in the release of the carboxyl-terminal amino acid residue.
The specificity of carboxypeptidase for carboxyl-terminal amino acid residues is due to the presence of a negatively charged pocket in the active site of the enzyme. This pocket attracts the positively charged amino group of the carboxyl-terminal amino acid residue, thereby positioning it for catalysis.
In summary, carboxypeptidase is an enzyme that hydrolyzes peptide bonds in proteins and peptides. It contains a zinc ion in its active site and is specific for carboxyl-terminal amino acid residues.
The mechanism of action of carboxypeptidase involves the binding of the substrate (a peptide or protein molecule) to the active site of the enzyme. The zinc ion acts as a Lewis acid, which means it accepts a pair of electrons from the peptide bond, thereby weakening the bond. This makes it easier for a water molecule to attack the peptide bond and break it, resulting in the release of the carboxyl-terminal amino acid residue.
The specificity of carboxypeptidase for carboxyl-terminal amino acid residues is due to the presence of a negatively charged pocket in the active site of the enzyme. This pocket attracts the positively charged amino group of the carboxyl-terminal amino acid residue, thereby positioning it for catalysis.
In summary, carboxypeptidase is an enzyme that hydrolyzes peptide bonds in proteins and peptides. It contains a zinc ion in its active site and is specific for carboxyl-terminal amino acid residues.
Chapter doubts & questions for Bioinorganic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Bioinorganic Chemistry - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup