All Exams >
BMAT >
Chemistry for BMAT (Section 2) >
All Questions
All questions of Atomic Structure for BMAT Exam
Which constituent of air is monoatomic?- a)Argon
- b)Water vapour
- c)Oxygen
- d)Nitrogen
Correct answer is option 'A'. Can you explain this answer?
Which constituent of air is monoatomic?
a)
Argon
b)
Water vapour
c)
Oxygen
d)
Nitrogen
|
|
Avinash Patel answered |
Argon is a Noble gas. Noble gases are not very reactive since they have a very stable electron configuration. It is only about 1% of the air in the atmosphere but it is more abundant than any other element on the Periodic Table behind Nitrogen and Oxygen.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following is the modern symbol of Gold?- a)Gl
- b)Go
- c)Si
- d)Au
Correct answer is option 'D'. Can you explain this answer?
Which one of the following is the modern symbol of Gold?
a)
Gl
b)
Go
c)
Si
d)
Au
|
|
Arvind Singh answered |
Gold is a chemical element with symbol Au (from Latin: aurum) and atomic number 79, making it one of the higher atomic number elements that occur naturally. In its purest form, it is a bright, slightly reddish yellow, dense, soft, malleable, and ductile metal.
The combining capacity of an element is called - a)Valency
- b)Atomicity
- c)Atomic number
- d)Valence electron
Correct answer is option 'A'. Can you explain this answer?
The combining capacity of an element is called
a)
Valency
b)
Atomicity
c)
Atomic number
d)
Valence electron
|
|
Aditi Sharma answered |
- The combining capacity of an element is known as its valency.
- Valency is the number of valence electrons in outer shell that take part in the chemical reaction.
Can you explain the answer of this question below:Which is the smallest particle of an element that can take part in a chemical reaction?
- A:
Molecule
- B:
Mixture
- C:
Compound
- D:
Atom
The answer is d.
Which is the smallest particle of an element that can take part in a chemical reaction?
Molecule
Mixture
Compound
Atom
|
|
Arvind Singh answered |
The basic unit of matter is the atom. It is the smallest particle of an element which can take part in chemical reactions and may or may not exist separately. It consists of sub-atomic particles, i.e., protons, neutrons and electrons.
What is the atomic mass of nitrogen?- a)16a.m.u.
- b)7a.m.u.
- c)14a.m.u.
- d)8 a.m.u.
Correct answer is option 'C'. Can you explain this answer?
What is the atomic mass of nitrogen?
a)
16a.m.u.
b)
7a.m.u.
c)
14a.m.u.
d)
8 a.m.u.

|
Anchal Singh answered |
Atomic Mass is determined by total number of nucleons in nucleus. Total number of nucleons is equal to sum of proton and neutrons in a atom .So, number of protons in Nitrogen is 7 as well as Neutron present are also 7. As 7 +7 is 14, the atomic mass is 14 u,(small u) is the atomic mass unit .
Identify the correct symbol of Gold. - a)Go
- b)Gd
- c)Ge
- d)Au
Correct answer is option 'D'. Can you explain this answer?
Identify the correct symbol of Gold.
a)
Go
b)
Gd
c)
Ge
d)
Au
|
|
Aditi Sharma answered |
The chemical symbol for gold is Au, taken from the Latin word for gold- Aurum. Its atomic number is 79.
The atom is indivisible, was proposed by- a)Einstein
- b)Lavoisier
- c)Dalton
- d)Proust
Correct answer is option 'C'. Can you explain this answer?
The atom is indivisible, was proposed by
a)
Einstein
b)
Lavoisier
c)
Dalton
d)
Proust

|
Deepnarayan Chauhan answered |
Yes john Dalton say this first but this was expect after because this is made up of electron protein and nuetron
One atomic mass unit is a mass unit equal to exactly one twelfth (1/12th) the mass of one atom of ___________.- a)Carbon-12
- b)Nitrogen -14
- c)Carbon-1
- d)4.Silicon-14
Correct answer is option 'A'. Can you explain this answer?
One atomic mass unit is a mass unit equal to exactly one twelfth (1/12th) the mass of one atom of ___________.
a)
Carbon-12
b)
Nitrogen -14
c)
Carbon-1
d)
4.Silicon-14
|
|
Jyoti Kapoor answered |
An atomic mass unit (symbolized AMU or amu) is defined as precisely 1/12 the mass of an atom of carbon-12. The carbon-12 (C-12) atom has six protons and six neutrons in its nucleus. In imprecise terms, one AMU is the average of the proton rest mass and the neutron rest mass.
Identify the symbol used to represent Avogadro’s number.- a)MA
- b)AO
- c)AN
- d)NA
Correct answer is option 'D'. Can you explain this answer?
Identify the symbol used to represent Avogadro’s number.
a)
MA
b)
AO
c)
AN
d)
NA
|
|
Arvind Singh answered |
Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022x10^23 mol-1 and is expressed as the symbol NA. Avogadro's number is a similar concept to that of a dozen or a gross.
NaCl molecule is made of which of the following ions?- a)Na cation and Cl anion
- b)Cl cation and Na anion
- c)Both Na and Cl cation
- d)Both Na and Cl anion
Correct answer is option 'A'. Can you explain this answer?
NaCl molecule is made of which of the following ions?
a)
Na cation and Cl anion
b)
Cl cation and Na anion
c)
Both Na and Cl cation
d)
Both Na and Cl anion
|
|
Jyoti Kapoor answered |
An oxygen molecule (O2) is a good example of a molecule with a covalent bond. Ionic bonds occur when electrons are donated from one atom to another. Table salt (NaCl) is a common example of a compound with an ionic bond.
If isotopic distribution of C-12 and C-14 are 98% and 2% respectively, then number of C-14 atoms in 12 g of carbon is
- a)1.032 x 1022
- b)6.886 × 1023
- c)3.88 x 1022
- d)1.302 x 1022
Correct answer is option 'A'. Can you explain this answer?
If isotopic distribution of C-12 and C-14 are 98% and 2% respectively, then number of C-14 atoms in 12 g of carbon is
a)
1.032 x 1022
b)
6.886 × 1023
c)
3.88 x 1022
d)
1.302 x 1022
|
|
Ananya Das answered |
In 12 g of carbon, the amount of C-14
= 12 × 2/100 = 0⋅24g
∴ C-14 atoms in 0.24g = (0⋅24×6⋅02×1023)/14
= 1⋅03×1022 atoms.
= 12 × 2/100 = 0⋅24g
∴ C-14 atoms in 0.24g = (0⋅24×6⋅02×1023)/14
= 1⋅03×1022 atoms.
The number of moles of oxygen atoms in two moles of nitric acid is:- a)2
- b)4
- c)8
- d)6
Correct answer is option 'D'. Can you explain this answer?
The number of moles of oxygen atoms in two moles of nitric acid is:
a)
2
b)
4
c)
8
d)
6

|
Deepshikha Mohanty answered |
Oxygen is 1/18th and A mole is a unit of measurement used to express the amount of substance contained in a certain amount of any given elemental chemical unit, such as atoms, molecules or ions. The number of these units contained in one mole of any substance is a constant, which is known as Avogadro’s number and is equal to 6.22x10^23 units. Moles and molecular mass play an essential role in calculating chemical reactions and the magnitudes involved in them.
Which one of the following phrases would be incorrect to use?- a)A mole of an element
- b)A mole of a compound
- c)An atom of an element
- d)An atom of a compound
Correct answer is option 'D'. Can you explain this answer?
Which one of the following phrases would be incorrect to use?
a)
A mole of an element
b)
A mole of a compound
c)
An atom of an element
d)
An atom of a compound
|
|
Krishna Iyer answered |
it will be incorrect to say that an atom of a compound because,
Every combination of atoms is a molecule. A compound is a molecule made of atoms from different elements. All compounds are molecules , but not all molecules are compounds.
Every combination of atoms is a molecule. A compound is a molecule made of atoms from different elements. All compounds are molecules , but not all molecules are compounds.
The number of atoms present in one molecule of an element is called as:- a)Empirical formula
- b)Molecule
- c)Atomicity
- d)Compound
Correct answer is option 'C'. Can you explain this answer?
The number of atoms present in one molecule of an element is called as:
a)
Empirical formula
b)
Molecule
c)
Atomicity
d)
Compound
|
|
Ananya Sharma answered |
Atomicity is the total number of atoms present in one molecule of an element or a compound.
For example: one molecule of hydrogen (H2) contains two atoms of hydrogen. Therefore, atomicity of hydrogen is 2. Similarly, 1 molecule of O3 contains 3 atoms. Therefore, its atomicity is 3. molecule of Argon exist as Ar and hence its a monoatomic compound.
Study of internal structure of Earth is known as- a)mechanics
- b)nuclear physics
- c)atomic physics
- d)geophysics
Correct answer is option 'D'. Can you explain this answer?
Study of internal structure of Earth is known as
a)
mechanics
b)
nuclear physics
c)
atomic physics
d)
geophysics
|
|
Sarita Reddy answered |
A geologist is a person who studies the earth. ... Geologists study the structure of the Earth, and they have found that it is made up of four main parts. The earth has a solid inner core, a liquid outer core, a mantle, and a crust.hence, option (D) is correct.
Cl2 stands for:- a)2 molecules of chlorine
- b)2 atoms of chlorine
- c)2 ions of chlorine
- d)3 molecule of chlorine
Correct answer is option 'B'. Can you explain this answer?
Cl2 stands for:
a)
2 molecules of chlorine
b)
2 atoms of chlorine
c)
2 ions of chlorine
d)
3 molecule of chlorine
|
|
Ravi Verma answered |
Cl’ represents one chlorine atom. It is found in nature in diatomic form as a molecule I.E. ,Cl2.
An atom has a mass number of 23 and atomic number 11. The number of protons are_________.- a)11
- b)12
- c)23
- d)44
Correct answer is option 'A'. Can you explain this answer?
An atom has a mass number of 23 and atomic number 11. The number of protons are_________.
a)
11
b)
12
c)
23
d)
44
|
|
Jyoti Kapoor answered |
Atomic number = 11, Mass number = 23, Number of protons - Atomic number = 11 Number of electrons = 11
Number of neutrons = Mass number- Atomic number Number of neutrons = 23 - 11 = 12
Valency of Calcium element is:- a)4
- b)5
- c)2
- d)3
Correct answer is option 'C'. Can you explain this answer?
Valency of Calcium element is:
a)
4
b)
5
c)
2
d)
3
|
|
Amit Sharma answered |
The valency of Calcium of 2+. Calcium has 2 electrons in its outermost shell, its electronic configuration being (2,8,8,2). In order to attain stability it loses 2 electrons to become stable according to the octet rule.
Which of the following is the chemical symbol for nitrogen gas?
- a)N2
- b)N
- c)N3
- d)Ni
Correct answer is option 'A'. Can you explain this answer?
Which of the following is the chemical symbol for nitrogen gas?
a)
N2
b)
N
c)
N3
d)
Ni
|
|
Mahi Chauhan answered |
The chemical formula of Nitrogen is N but Nitrogen exists in the molecule of two ions hence the chemical symbol of Nitrogen gas is written as N2.
Which atom is the smallest atom of all?- a)Lithium
- b)Helium
- c)Hydrogen
- d)Carbon
Correct answer is option 'C'. Can you explain this answer?
Which atom is the smallest atom of all?
a)
Lithium
b)
Helium
c)
Hydrogen
d)
Carbon
|
|
Gayatri Deshpande answered |
Explanation:
Factors Affecting Atomic Radius:
Comparison of Atomic Radii:
Conclusion:
The size of an atom is determined by its atomic radius, which is the distance from the center of the nucleus to the outermost electron. Therefore, the smallest atom will have the smallest atomic radius.
Factors Affecting Atomic Radius:
- Number of protons in the nucleus
- Number of energy levels or electron shells
- Effective nuclear charge
Comparison of Atomic Radii:
Based on the factors affecting atomic radius, we can compare the atomic radii of the four given elements:
- Hydrogen has only one proton and one electron, making it the smallest atom.
- Helium has two protons and two electrons, making it slightly larger than hydrogen.
- Lithium has three protons and electrons, and is larger than both hydrogen and helium.
- Carbon has six protons and electrons, and is larger than all three previous elements.
Conclusion:
Therefore, the smallest atom of all is Hydrogen, with only one proton and one electron.
How do atoms usually exist in nature?- a)In the form of molecules
- b)In the form of ions
- c)In the free state
- d)In the form of molecules and Ions
Correct answer is option 'D'. Can you explain this answer?
How do atoms usually exist in nature?
a)
In the form of molecules
b)
In the form of ions
c)
In the free state
d)
In the form of molecules and Ions

|
Khushi answered |
Most of the elements in environment do not have 8
electrons in their valance shells(because of which they are more or less reactive and unstable ) so they react with the atoms of other metals to form molecules or ion to achieve noble gas configuration and become stable. The atoms of only a few elements like helium , Neon ..etc have their valance shells already filled with 8 electrons.so they can exist in free state. But matter mostly exist in the form of molecules and ions.
Identify the correct one from the following statements. - a)Elements can be changed into simpler substances.
- b)Compound has variable composition
- c)The mixture has properties similar to its components.
- d)Brass is a compound of Copper and Zinc
Correct answer is option 'C'. Can you explain this answer?
Identify the correct one from the following statements.
a)
Elements can be changed into simpler substances.
b)
Compound has variable composition
c)
The mixture has properties similar to its components.
d)
Brass is a compound of Copper and Zinc
|
|
Geetika Shah answered |
option ( c) The mixture has properties similar to its compound is the correct answer.
Explanation:-
This is because in a mixture, the constituent particles are not mixed chemically.
They are mixed physically and can be separated fairly easily by physical Methods.
Thus they show the properties of components.
Example can be alloys.
They are mixed physically and can be separated fairly easily by physical Methods.
Thus they show the properties of components.
Example can be alloys.
If 12 grams of carbon has n atoms, then the number of atoms in 12 grams of magnesium will be:- a)2n
- b)3n
- c)n/2
- d)n
Correct answer is option 'C'. Can you explain this answer?
If 12 grams of carbon has n atoms, then the number of atoms in 12 grams of magnesium will be:
a)
2n
b)
3n
c)
n/2
d)
n
|
|
Rohit Sharma answered |
C = 12 g = 1 mole
1 mole = 24 g = n atoms
1 g = (n * 12) / 24
1 g = n /2
1 mole = 24 g = n atoms
1 g = (n * 12) / 24
1 g = n /2
1.0 mole CO2 contains- a)6.022 x 10-23 atoms of carbon
- b)6.022 X 10-23 atoms of oxygen
- c)6.022 x 1023 molecules of CO2
- d)3g atom of CO2
Correct answer is option 'C'. Can you explain this answer?
1.0 mole CO2 contains
a)
6.022 x 10-23 atoms of carbon
b)
6.022 X 10-23 atoms of oxygen
c)
6.022 x 1023 molecules of CO2
d)
3g atom of CO2
|
|
Swati Verma answered |
- One mole of CO2 = one mole of C = 6.02×10 23 atoms of carbon.
- One mole of CO2 = two moles of O = 2×6.02×1023= 12.04×10 23 atoms of oxygen.
- One mole of CO2 = 6.02×1023 molecules of CO2
Note: 6.02×1023 is Avogadro's number and represents number of atoms/molecules present in one mole of substance.
Which of the following symbols is incorrect?
(i) Helium (He)
(ii) Aluminium (AL)
(iii) Carbon (c)
(iv) Cobalt (CO)
Select the correct alternative:
- a)(i) and (ii)
- b)(i), (ii) and (iii)
- c)(ii), (iii) and (iv)
- d)(i), (iii) and (iv)
Correct answer is option 'C'. Can you explain this answer?
Which of the following symbols is incorrect?
(i) Helium (He)
(ii) Aluminium (AL)
(iii) Carbon (c)
(iv) Cobalt (CO)
Select the correct alternative:
(i) Helium (He)
(ii) Aluminium (AL)
(iii) Carbon (c)
(iv) Cobalt (CO)
Select the correct alternative:
a)
(i) and (ii)
b)
(i), (ii) and (iii)
c)
(ii), (iii) and (iv)
d)
(i), (iii) and (iv)
|
|
Ananya Das answered |
Because , the symbol used for aluminum is Al, the symbol used for carbon is C, the symbol used for cobalt is Co.
Calculate the relative molecular mass of H2S.- a)16 a.m.u.
- b)64 a.m.u.
- c)18 a.m.u.
- d)34 a.m.u.
Correct answer is option 'D'. Can you explain this answer?
Calculate the relative molecular mass of H2S.
a)
16 a.m.u.
b)
64 a.m.u.
c)
18 a.m.u.
d)
34 a.m.u.
|
|
Vikram Khanna answered |
Atomic mass of hydrogen = 1 a.m.u.
Atomic mass of Sulphur = 32 a.m.u.
Thus, the molecular mass of hydrogen sulphide, which contains two atoms of hydrogen and one atom of sulphur is = 2 X 1 + 1 X 32 = 34 a.m.u.
The atomic mass of calcium is 40. Calculate the number of moles in 16 grams of calcium.- a)0.4 mole
- b)4 mole
- c)1/4 mole
- d)640 mole
Correct answer is option 'A'. Can you explain this answer?
The atomic mass of calcium is 40. Calculate the number of moles in 16 grams of calcium.
a)
0.4 mole
b)
4 mole
c)
1/4 mole
d)
640 mole
|
|
Vikram Khanna answered |
atomic mass of calcium is 40u.
No. of moles =given weight/molecular weight
No. of Cu moles=0.4/40=1/00=0.01moles
We know that 1 mole of any substance contains 6.023 x 10^23 atoms/ molecules/ions
1mole of Cu contains=6.023 x 10^23 atoms of Cu
0.01 mole of Cu contains =6.023 x10^21 atoms of calcium.
∴The no. of calcium atoms in 0.4 u of calcium are 6.023 x10^21 atoms
If atomic mass of sodium is 23 a.m.u., then the mass of 3.01 x 1023 sodium atoms is:- a)11.5 kg
- b)23 g
- c)0.5 mol
- d)11.5 g
Correct answer is option 'D'. Can you explain this answer?
If atomic mass of sodium is 23 a.m.u., then the mass of 3.01 x 1023 sodium atoms is:
a)
11.5 kg
b)
23 g
c)
0.5 mol
d)
11.5 g
|
|
Naina Sharma answered |
Mass of 6.022 X 10 23 atoms (1 mole) of sodium = 23 g

1 mole of water molecules has:- a)2 moles of oxygen atoms
- b)1 mole of hydrogen atom
- c)2 moles of hydrogen atoms
- d)2 moles of hydrogen molecule
Correct answer is option 'C'. Can you explain this answer?
1 mole of water molecules has:
a)
2 moles of oxygen atoms
b)
1 mole of hydrogen atom
c)
2 moles of hydrogen atoms
d)
2 moles of hydrogen molecule
|
|
Arvind Singh answered |
So, 1 mole H2O = 1.2044X10^24 hydrogen atoms. Therefore 2 mole H2O will have 2.4088X10^24 hydrogen atoms. Since one mole is defined as 6.022 x 10^23, and there are two hydrogen per molecule, that would mean that there are exactly 12.044 x 10^23 molecules of hydrogen in one mole of water.
Can you explain the answer of this question below:Which of the following pair of elements represents a mole ratio of 1:1?
- A:
20 g of sodium and 20 g of calcium
- B:
7 g of nitrogen and 12 gm of sodium
- C:
10 g of calcium and 6 g of carbon
- D:
14 g of nitrogen and 24 g of magnesium
The answer is d.
Which of the following pair of elements represents a mole ratio of 1:1?
20 g of sodium and 20 g of calcium
7 g of nitrogen and 12 gm of sodium
10 g of calcium and 6 g of carbon
14 g of nitrogen and 24 g of magnesium
|
|
Jyoti Kapoor answered |
14 g of nitrogen = gram atomic mass of nitrogen = 1 mole of nitrogen atoms24 g of magnesium = gram atomic mass of magnesium = 1 mole of magnesium atomsSo, mole ratio = 1:1
How many atoms does one mole of Phosphorus contain?- a)2× 6.022 × 1023 atoms
- b)3× 6.022 × 1023 atoms
- c)6.022 × 1023 atoms
- d)4 × 6.022 × 1023 atoms
Correct answer is option 'D'. Can you explain this answer?
How many atoms does one mole of Phosphorus contain?
a)
2× 6.022 × 1023 atoms
b)
3× 6.022 × 1023 atoms
c)
6.022 × 1023 atoms
d)
4 × 6.022 × 1023 atoms
|
|
Arvind Singh answered |
No of atom in 1 mole = 6.022*10^23
No of atom in 1 mole of P4 = 4* 6.022*10^23
The mass of 0.5 moles of Water molecules is:(Atomic mass, H =1; O = 16)- a)27 g
- b)0.5 g
- c)9 g
- d)18 g
Correct answer is option 'C'. Can you explain this answer?
The mass of 0.5 moles of Water molecules is:(Atomic mass, H =1; O = 16)
a)
27 g
b)
0.5 g
c)
9 g
d)
18 g
|
|
Arvind Singh answered |
For 1 mole of water =molecular mass of water (H2O)in grams
=mass of 2H atoms +mass of O atoms
=2x1+16
=mass of 2H atoms +mass of O atoms
=2x1+16
=2+16
=18 grams ⇒ for 1 mole
For 0.5 moles = 18x0.5
= 9.0 grams
Valency of hydrogen is 1 and that of sulphur is 2. What should be the formula of hydrogen sulphide?- a)HS
- b)H2S2
- c)HS2
- d)H2S
Correct answer is option 'D'. Can you explain this answer?
Valency of hydrogen is 1 and that of sulphur is 2. What should be the formula of hydrogen sulphide?
a)
HS
b)
H2S2
c)
HS2
d)
H2S
|
|
Jyoti Kapoor answered |
Hydrogen sulfide is the chemical compound with the chemical formula H2S. It is a colorless gas with the characteristic foul odor of rotten eggs. It is very poisonous, corrosive, and flammable.
The percentage of hydrogen in water is______.
- a)1.11%
- b)11.11%
- c)8.89 %
- d)88.9%
Correct answer is option 'B'. Can you explain this answer?
The percentage of hydrogen in water is______.
a)
1.11%
b)
11.11%
c)
8.89 %
d)
88.9%
|
|
Arun Sharma answered |
There is also one mole of oxygen atoms weighing 16.00 grams in the mole of water and that of hydrogen is 2.00 gram.
To get the percentage of hydrogen, divide the 2 by 18 and multiply by 100.
i.e. Percentage of hydrogen = (2/18) x 100 = 11.11%
To get the percentage of hydrogen, divide the 2 by 18 and multiply by 100.
i.e. Percentage of hydrogen = (2/18) x 100 = 11.11%
Which of the following can be used to see atoms?- a)Scanning tunneling microscope
- b)Microscope
- c)Most powerful microscope
- d)Optical microscope
Correct answer is option 'A'. Can you explain this answer?
Which of the following can be used to see atoms?
a)
Scanning tunneling microscope
b)
Microscope
c)
Most powerful microscope
d)
Optical microscope
|
|
Jyoti Kapoor answered |
A scanning tunneling microscope (STM) is an instrument for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer (at IBM Zrich), the Nobel Prize in Physics in 1986. For an STM, good resolution is considered to be 0.1 nm lateral resolution and 0.01 nm (10 pm) depth resolution.With this resolution, individual atoms within materials are routinely imaged and manipulated. The STM can be used not only in ultra-high vacuum but also in air, water, and various other liquid or gas ambients, and at temperatures ranging from near zero kelvin to over 1000 DEGC.
Molecules can exist in free state because:- a)They are very stable.
- b)They are electrically charged.
- c)They are very reactive.
- d)They are bigger in size as compared to atoms.
Correct answer is option 'A'. Can you explain this answer?
Molecules can exist in free state because:
a)
They are very stable.
b)
They are electrically charged.
c)
They are very reactive.
d)
They are bigger in size as compared to atoms.

|
Raghavendra Jain answered |
The answer to the given question is option 'A': Molecules can exist in a free state because they are very stable.
Explanation:
Molecules are formed when two or more atoms chemically combine by sharing electrons or through other types of bonding. These atoms can be from the same element or different elements. The resulting molecule has a stable structure due to the sharing or transfer of electrons, which allows the atoms to achieve a more stable electron configuration. Here is a detailed explanation of why molecules are stable and can exist in a free state:
1. Stable electron configuration:
Molecules are formed when atoms combine in such a way that they achieve a stable electron configuration. This stability is achieved by filling or emptying their outermost energy level, also known as the valence shell. By sharing or transferring electrons, atoms can achieve a full valence shell, which is a highly stable configuration. This stable electron configuration contributes to the stability of molecules.
2. Strong chemical bonds:
Molecules are held together by strong chemical bonds, such as covalent bonds or ionic bonds. Covalent bonds involve the sharing of electrons between atoms, while ionic bonds involve the transfer of electrons from one atom to another. These bonds are formed through the attraction between positively charged nuclei and negatively charged electrons. The strength of these bonds contributes to the stability of molecules.
3. Low potential energy:
Molecules have lower potential energy compared to individual atoms. When atoms combine to form a molecule, the resulting structure has a lower energy state. This decrease in potential energy is due to the formation of chemical bonds, which release energy. The lower potential energy of molecules makes them more stable and allows them to exist in a free state.
4. Balanced forces:
In a molecule, the attractive forces between atoms (chemical bonds) are balanced by repulsive forces between electrons and between nuclei. This balance of forces helps maintain the structure of the molecule and prevents it from easily breaking apart. The balanced forces contribute to the stability of molecules in a free state.
Conclusion:
In conclusion, molecules can exist in a free state because they are very stable. This stability is due to their stable electron configuration, strong chemical bonds, low potential energy, and balanced forces. These factors work together to maintain the integrity of the molecule and prevent it from easily dissociating into its constituent atoms.
Explanation:
Molecules are formed when two or more atoms chemically combine by sharing electrons or through other types of bonding. These atoms can be from the same element or different elements. The resulting molecule has a stable structure due to the sharing or transfer of electrons, which allows the atoms to achieve a more stable electron configuration. Here is a detailed explanation of why molecules are stable and can exist in a free state:
1. Stable electron configuration:
Molecules are formed when atoms combine in such a way that they achieve a stable electron configuration. This stability is achieved by filling or emptying their outermost energy level, also known as the valence shell. By sharing or transferring electrons, atoms can achieve a full valence shell, which is a highly stable configuration. This stable electron configuration contributes to the stability of molecules.
2. Strong chemical bonds:
Molecules are held together by strong chemical bonds, such as covalent bonds or ionic bonds. Covalent bonds involve the sharing of electrons between atoms, while ionic bonds involve the transfer of electrons from one atom to another. These bonds are formed through the attraction between positively charged nuclei and negatively charged electrons. The strength of these bonds contributes to the stability of molecules.
3. Low potential energy:
Molecules have lower potential energy compared to individual atoms. When atoms combine to form a molecule, the resulting structure has a lower energy state. This decrease in potential energy is due to the formation of chemical bonds, which release energy. The lower potential energy of molecules makes them more stable and allows them to exist in a free state.
4. Balanced forces:
In a molecule, the attractive forces between atoms (chemical bonds) are balanced by repulsive forces between electrons and between nuclei. This balance of forces helps maintain the structure of the molecule and prevents it from easily breaking apart. The balanced forces contribute to the stability of molecules in a free state.
Conclusion:
In conclusion, molecules can exist in a free state because they are very stable. This stability is due to their stable electron configuration, strong chemical bonds, low potential energy, and balanced forces. These factors work together to maintain the integrity of the molecule and prevent it from easily dissociating into its constituent atoms.
Chemical formula of a compound is A2B3. The valency of A is- a)3
- b)6
- c)2
- d)5
Correct answer is option 'A'. Can you explain this answer?
Chemical formula of a compound is A2B3. The valency of A is
a)
3
b)
6
c)
2
d)
5
|
|
Anita Menon answered |
The valency of A in A2B3 is 3.
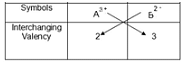

Can you explain the answer of this question below:Which of the following is correct pair of elements and its symbol?
- A:
Silver – Si
- B:
Sodium – So
- C:
Potassium – Pt
- D:
Sulphur – S
The answer is d.
Which of the following is correct pair of elements and its symbol?
Silver – Si
Sodium – So
Potassium – Pt
Sulphur – S

|
Prachi Rathore answered |
Here are the righ pairs .......... ... silver-Ag ....... sodium-Na ...... potassium- K...... and sulphur - s so in the given options (D) sulphur - s. is right answer
What is the value of Avogadro’s number?- a)12.044 x 1022
- b)6.022 x 1023
- c)3.011 x 1023
- d)6.022 x 1022
Correct answer is option 'B'. Can you explain this answer?
What is the value of Avogadro’s number?
a)
12.044 x 1022
b)
6.022 x 1023
c)
3.011 x 1023
d)
6.022 x 1022

|
Arvi Singh Oberoi. answered |
This is 6.022×10 power 23.....
1mole=6.022 ×10 power 23.....
ya...
1mole=6.022 ×10 power 23.....
ya...
One mole of molecules may not be equal to:- a)6.022 x 1023 number of molecules
- b)Given number of particles (N)
- c)Avogadro number
- d)Molecular mass in grams
Correct answer is option 'B'. Can you explain this answer?
One mole of molecules may not be equal to:
a)
6.022 x 1023 number of molecules
b)
Given number of particles (N)
c)
Avogadro number
d)
Molecular mass in grams
|
|
Ananya Sharma answered |
- The mole allows scientists to calculate the number of elementary entities (usually atoms or molecules) in a certain mass of a given substance.
- Avogadro’s number is an absolute number: there are 6.022x10^23 elementary entities in 1 mole. This can also be written as 6.022x10^23 mol-1.
- The mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams.
18 g of water is electrolysed. The weight of oxygen formed will be - a)16 g
- b)8 g
- c)4 g
- d)2 g
Correct answer is option 'A'. Can you explain this answer?
18 g of water is electrolysed. The weight of oxygen formed will be
a)
16 g
b)
8 g
c)
4 g
d)
2 g
|
|
Parth Khanna answered |
Electrolysis of Water
Electrolysis is a process of decomposition of a compound by passing electricity through it. Water can also be electrolyzed to produce hydrogen and oxygen gases.
The balanced chemical equation for the electrolysis of water is as follows:
2H2O(l) → 2H2(g) + O2(g)
From this equation, it is clear that for every 2 moles of water electrolyzed, 1 mole of oxygen gas is produced.
Calculation of Oxygen Gas Produced
Given that 18 g of water is electrolyzed, we need to calculate the weight of oxygen gas produced.
The molar mass of water (H2O) is 18 g/mol. Therefore, 18 g of water is equal to 1 mole of water.
According to the balanced chemical equation, 1 mole of water produces 1/2 mole of oxygen gas.
The molar mass of oxygen (O2) is 32 g/mol. Therefore, 1/2 mole of oxygen gas is equal to 16 g.
Hence, the weight of oxygen gas produced from the electrolysis of 18 g of water is 16 g.
Therefore, option A, 16 g, is the correct answer.
Electrolysis is a process of decomposition of a compound by passing electricity through it. Water can also be electrolyzed to produce hydrogen and oxygen gases.
The balanced chemical equation for the electrolysis of water is as follows:
2H2O(l) → 2H2(g) + O2(g)
From this equation, it is clear that for every 2 moles of water electrolyzed, 1 mole of oxygen gas is produced.
Calculation of Oxygen Gas Produced
Given that 18 g of water is electrolyzed, we need to calculate the weight of oxygen gas produced.
The molar mass of water (H2O) is 18 g/mol. Therefore, 18 g of water is equal to 1 mole of water.
According to the balanced chemical equation, 1 mole of water produces 1/2 mole of oxygen gas.
The molar mass of oxygen (O2) is 32 g/mol. Therefore, 1/2 mole of oxygen gas is equal to 16 g.
Hence, the weight of oxygen gas produced from the electrolysis of 18 g of water is 16 g.
Therefore, option A, 16 g, is the correct answer.
Which of the following represents a correct chemical formula?- a)CaCI
- b)NaS04
- c)NaS
- d)NaCI
Correct answer is option 'D'. Can you explain this answer?
Which of the following represents a correct chemical formula?
a)
CaCI
b)
NaS04
c)
NaS
d)
NaCI
|
|
Pratishtha Ranjan answered |
Absolutely NaCl is the right answer because it is the formula of Sodium Chloride (salt)
A compound contains 8% sulphur by mass. The minimum molecular weight of the compound is:
- a)100
- b)200
- c)300
- d)400
Correct answer is option 'D'. Can you explain this answer?
A compound contains 8% sulphur by mass. The minimum molecular weight of the compound is:
a)
100
b)
200
c)
300
d)
400
|
|
Alok Sharma answered |
Solution:
Given, the compound contains 8% sulphur by mass.
Let us assume the molecular mass of the compound to be x.
Then, the mass of sulphur in the compound = 8% of x = (8/100)x = 0.08x
As we know, the atomic mass of sulphur is 32u.
So, the number of moles of sulphur in the compound = (0.08x)/32 = 0.0025x
Now, let us assume that the compound contains only one sulphur atom.
Then, the number of moles of the compound = 0.0025x
And, the molecular weight of the compound = x
Now, we can write:
x = (mass of sulphur/molar mass of sulphur) + (mass of other atoms/molar mass of other atoms)
x = (0.08x/32) + (mass of other atoms/molar mass of other atoms)
x - (0.08x/32) = mass of other atoms/molar mass of other atoms
31x/32 = mass of other atoms/molar mass of other atoms
molar mass of the compound = mass of other atoms/(31x/32)
We know that the molecular weight of the compound is always greater than or equal to its molar mass.
Therefore, the minimum molecular weight of the compound = (molar mass of the compound) × (number of moles of the compound)
= [(mass of other atoms/(31x/32)) × 0.0025x]
= (mass of other atoms/31) × 0.025
Now, the question is to find out the minimum molecular weight of the compound.
Since we have assumed that the compound contains only one sulphur atom, the mass of other atoms in the compound = (100 - 8)% = 92%
Therefore, the minimum molecular weight of the compound = (mass of other atoms/31) × 0.025
= (92/31) × 0.025
= 0.076
= 400
Hence, the correct option is (d) 400.
Given, the compound contains 8% sulphur by mass.
Let us assume the molecular mass of the compound to be x.
Then, the mass of sulphur in the compound = 8% of x = (8/100)x = 0.08x
As we know, the atomic mass of sulphur is 32u.
So, the number of moles of sulphur in the compound = (0.08x)/32 = 0.0025x
Now, let us assume that the compound contains only one sulphur atom.
Then, the number of moles of the compound = 0.0025x
And, the molecular weight of the compound = x
Now, we can write:
x = (mass of sulphur/molar mass of sulphur) + (mass of other atoms/molar mass of other atoms)
x = (0.08x/32) + (mass of other atoms/molar mass of other atoms)
x - (0.08x/32) = mass of other atoms/molar mass of other atoms
31x/32 = mass of other atoms/molar mass of other atoms
molar mass of the compound = mass of other atoms/(31x/32)
We know that the molecular weight of the compound is always greater than or equal to its molar mass.
Therefore, the minimum molecular weight of the compound = (molar mass of the compound) × (number of moles of the compound)
= [(mass of other atoms/(31x/32)) × 0.0025x]
= (mass of other atoms/31) × 0.025
Now, the question is to find out the minimum molecular weight of the compound.
Since we have assumed that the compound contains only one sulphur atom, the mass of other atoms in the compound = (100 - 8)% = 92%
Therefore, the minimum molecular weight of the compound = (mass of other atoms/31) × 0.025
= (92/31) × 0.025
= 0.076
= 400
Hence, the correct option is (d) 400.
The mass of one atom of carbon-12 is- a)1 g
- b)1.99 x 10-23g
- c)1/12 g
- d)1.99 x 1023 g
Correct answer is option 'B'. Can you explain this answer?
The mass of one atom of carbon-12 is
a)
1 g
b)
1.99 x 10-23g
c)
1/12 g
d)
1.99 x 1023 g
|
|
Ritika Chauhan answered |
Mass of one atom = Avogadro′s numberMass of one mole of atom=12/6.02×1023
Mass of one carbon atom = 1.994×10−23g
Chapter doubts & questions for Atomic Structure - Chemistry for BMAT (Section 2) 2024 is part of BMAT exam preparation. The chapters have been prepared according to the BMAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for BMAT 2024 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Atomic Structure - Chemistry for BMAT (Section 2) in English & Hindi are available as part of BMAT exam.
Download more important topics, notes, lectures and mock test series for BMAT Exam by signing up for free.
Chemistry for BMAT (Section 2)
146 videos|126 docs|121 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup