JEE Exam > JEE Questions > Resonance occurs due to thea)delocalization o...
Start Learning for Free
Resonance occurs due to the
- a)delocalization of a lone pair of electrons
- b)delocalization of sigma electrons
- c)delocalization of pi electrons
- d)migration of protons
Correct answer is option 'A,C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Resonance occurs due to thea)delocalization of a lone pair of electron...
Free Test
FREE
| Start Free Test |
Community Answer
Resonance occurs due to thea)delocalization of a lone pair of electron...
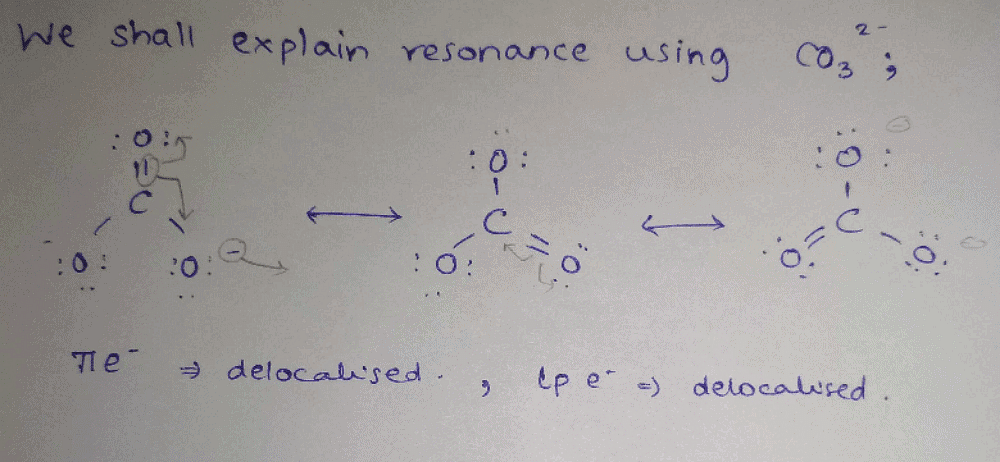
Understanding Resonance
Resonance is a fundamental concept in chemistry that describes the delocalization of electrons within molecules. It helps explain the stability and reactivity of certain compounds.
Delocalization of Electrons
- Resonance primarily involves the delocalization of pi electrons.
- In molecules with conjugated systems, such as benzene, the pi electrons can move freely across adjacent p-orbitals, creating a stable structure that is a hybrid of multiple contributing structures.
Lone Pairs and Sigma Electrons
- Delocalization of a lone pair of electrons can also contribute to resonance.
- For example, in certain aromatic compounds, a lone pair of electrons from a heteroatom can interact with the pi system, enhancing stability.
Why Sigma Electrons Are Not Involved
- Delocalization of sigma electrons does not contribute to resonance.
- Sigma bonds are localized between two atoms and do not participate in resonance structures. They maintain a fixed position as they result from the head-on overlap of atomic orbitals.
Conclusion
- The correct answer to the question about resonance is options 'A' and 'C', as they correctly identify the roles of lone pair electrons and pi electrons in resonance structures.
- Understanding these principles is crucial for predicting molecular behavior in various chemical reactions, particularly in organic chemistry, which is vital for exams like JEE.
Resonance is a fundamental concept in chemistry that describes the delocalization of electrons within molecules. It helps explain the stability and reactivity of certain compounds.
Delocalization of Electrons
- Resonance primarily involves the delocalization of pi electrons.
- In molecules with conjugated systems, such as benzene, the pi electrons can move freely across adjacent p-orbitals, creating a stable structure that is a hybrid of multiple contributing structures.
Lone Pairs and Sigma Electrons
- Delocalization of a lone pair of electrons can also contribute to resonance.
- For example, in certain aromatic compounds, a lone pair of electrons from a heteroatom can interact with the pi system, enhancing stability.
Why Sigma Electrons Are Not Involved
- Delocalization of sigma electrons does not contribute to resonance.
- Sigma bonds are localized between two atoms and do not participate in resonance structures. They maintain a fixed position as they result from the head-on overlap of atomic orbitals.
Conclusion
- The correct answer to the question about resonance is options 'A' and 'C', as they correctly identify the roles of lone pair electrons and pi electrons in resonance structures.
- Understanding these principles is crucial for predicting molecular behavior in various chemical reactions, particularly in organic chemistry, which is vital for exams like JEE.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer?
Question Description
Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer?.
Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Resonance occurs due to thea)delocalization of a lone pair of electronsb)delocalization of sigma electronsc)delocalization of pi electronsd)migration of protonsCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























