Class 12 Exam > Class 12 Questions > An organic compound X (C5H12O) gives efferves...
Start Learning for Free
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X is
- a)It is 2-pentanol
- b)It is 3-pentanol
- c)With HBr, it gives achiral alkyl bromide
- d)The oxidation product C5H10O2 is achiral
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
An organic compound X (C5H12O) gives effervescence with NaH. X on trea...
X is a primary, chiral alcohol.
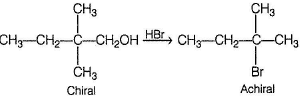
Most Upvoted Answer
An organic compound X (C5H12O) gives effervescence with NaH. X on trea...
**Explanation:**
To determine the correct statement regarding compound X, let's analyze the given information step by step.
**1. Compound X gives effervescence with NaH.**
Effervescence with NaH indicates the presence of an acidic proton in compound X. This suggests that compound X contains a hydroxyl group (-OH).
**2. Treatment of X with acidic CrO3 solution turns the solution blue-green and forms C5H10O2.**
The reaction of compound X with acidic CrO3 is a common test for the presence of a double bond. The formation of C5H10O2 suggests that compound X contains a double bond, and the CrO3 oxidation has converted the double bond into a carbonyl group.
**3. Compound X can be resolved into enantiomers.**
Resolution of a compound into enantiomers indicates the presence of a chiral center. A chiral center is a carbon atom bonded to four different groups. In the case of compound X, it must contain at least one carbon atom with four different substituents.
Now, let's analyze each option based on the given information:
**a) It is 2-pentanol**
2-Pentanol has the molecular formula C5H12O, which matches the molecular formula of compound X. However, 2-pentanol does not contain a double bond or a chiral center. Therefore, option a) is incorrect.
**b) It is 3-pentanol**
3-Pentanol also has the molecular formula C5H12O, but it lacks a double bond and a chiral center. Therefore, option b) is incorrect.
**c) With HBr, it gives an achiral alkyl bromide**
This statement suggests that compound X does not contain a chiral center, as an achiral alkyl bromide does not have a chiral center. This matches the given information that compound X can be resolved into enantiomers. Therefore, option c) is correct.
**d) The oxidation product C5H10O2 is achiral**
The formation of a chiral compound from compound X indicates that compound X itself must contain a chiral center. Therefore, the oxidation product C5H10O2 cannot be achiral. Therefore, option d) is incorrect.
In conclusion, the correct statement regarding compound X is option c) - "With HBr, it gives an achiral alkyl bromide."
To determine the correct statement regarding compound X, let's analyze the given information step by step.
**1. Compound X gives effervescence with NaH.**
Effervescence with NaH indicates the presence of an acidic proton in compound X. This suggests that compound X contains a hydroxyl group (-OH).
**2. Treatment of X with acidic CrO3 solution turns the solution blue-green and forms C5H10O2.**
The reaction of compound X with acidic CrO3 is a common test for the presence of a double bond. The formation of C5H10O2 suggests that compound X contains a double bond, and the CrO3 oxidation has converted the double bond into a carbonyl group.
**3. Compound X can be resolved into enantiomers.**
Resolution of a compound into enantiomers indicates the presence of a chiral center. A chiral center is a carbon atom bonded to four different groups. In the case of compound X, it must contain at least one carbon atom with four different substituents.
Now, let's analyze each option based on the given information:
**a) It is 2-pentanol**
2-Pentanol has the molecular formula C5H12O, which matches the molecular formula of compound X. However, 2-pentanol does not contain a double bond or a chiral center. Therefore, option a) is incorrect.
**b) It is 3-pentanol**
3-Pentanol also has the molecular formula C5H12O, but it lacks a double bond and a chiral center. Therefore, option b) is incorrect.
**c) With HBr, it gives an achiral alkyl bromide**
This statement suggests that compound X does not contain a chiral center, as an achiral alkyl bromide does not have a chiral center. This matches the given information that compound X can be resolved into enantiomers. Therefore, option c) is correct.
**d) The oxidation product C5H10O2 is achiral**
The formation of a chiral compound from compound X indicates that compound X itself must contain a chiral center. Therefore, the oxidation product C5H10O2 cannot be achiral. Therefore, option d) is incorrect.
In conclusion, the correct statement regarding compound X is option c) - "With HBr, it gives an achiral alkyl bromide."

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer?
Question Description
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer?.
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer?.
Solutions for An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice An organic compound X (C5H12O) gives effervescence with NaH. X on treatment with acidic CrO3 solution turns solution to blue-green forming C5H10O2. Also X can be resolved into enantiomers. The correct statement regarding X isa)It is 2-pentanolb)It is 3-pentanolc)With HBr, it gives achiral alkyl bromided)The oxidation product C5H10O2 is achiralCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.













