IIT JAM Exam > IIT JAM Questions > How-to Extract uranium from pitch blende with...
Start Learning for Free
How-to Extract uranium from pitch blende with equation ?
Most Upvoted Answer
How-to Extract uranium from pitch blende with equation ?
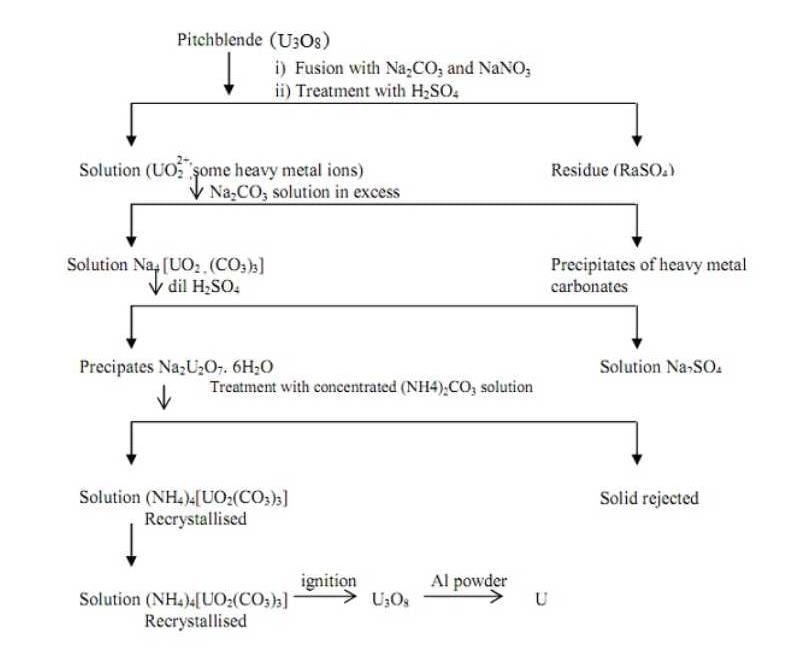
Extracting Uranium from Pitchblende
Pitchblende is a mineral that contains significant amounts of uranium. Extracting uranium from pitchblende involves several steps, including crushing and grinding the ore, chemical processing, and purification. Below is a detailed guide on how to extract uranium from pitchblende, along with the relevant equations involved in the process.
1. Crushing and Grinding
Pitchblende is first crushed into small pieces and then ground into a fine powder. This step increases the surface area of the ore, allowing for better chemical reactions in subsequent processes.
2. Leaching
Leaching is the process of dissolving the uranium from the powdered pitchblende ore using a suitable chemical solution. The most commonly used leaching agent is sulfuric acid (H2SO4), which reacts with the uranium minerals to form soluble uranium sulfate (UO2SO4).
The chemical equation for leaching uranium from pitchblende can be represented as follows:
U3O8 (pitchblende) + 4H2SO4 (sulfuric acid) → 3UO2SO4 (uranium sulfate) + 4H2O (water)
3. Filtration
After leaching, the resulting solution containing uranium sulfate is separated from the solid residue (gangue) through filtration. Filtration helps remove impurities and other insoluble materials.
4. Purification
The uranium sulfate solution obtained from filtration still contains impurities that need to be removed. One common method for purification is solvent extraction, which involves using an organic solvent to selectively extract uranium from the solution.
5. Precipitation
To obtain solid uranium compounds, the purified uranium solution is further processed through precipitation. Ammonium diuranate (NH4U2O7) is commonly formed by adding ammonium hydroxide (NH4OH) or ammonium carbonate (NH4)2CO3 to the solution. The precipitate is then collected, washed, and dried.
The chemical equation for precipitation of ammonium diuranate can be represented as follows:
UO2SO4 (uranium sulfate) + 2NH4OH (ammonium hydroxide) → NH4U2O7 (ammonium diuranate) + H2SO4 (sulfuric acid) + H2O (water)
6. Conversion to Uranium Oxide
The ammonium diuranate obtained from precipitation is converted into uranium oxide (U3O8) through calcination. Calcination involves heating the ammonium diuranate at high temperatures to remove the remaining impurities and transform it into a more stable oxide form.
The chemical equation for the conversion of ammonium diuranate to uranium oxide can be represented as follows:
2(NH4)2U2O7 (ammonium diuranate) → U3O8 (uranium oxide) + 4NH3 (ammonia) + 3H2O (water)
7. Final Purification
The uranium oxide is further purified using various techniques like solvent extraction, ion exchange, or precipitation to ensure its quality and remove any remaining impurities.
Conclusion
Extracting uranium from pitchblende involves several stages, including crushing and grinding the ore, le
Pitchblende is a mineral that contains significant amounts of uranium. Extracting uranium from pitchblende involves several steps, including crushing and grinding the ore, chemical processing, and purification. Below is a detailed guide on how to extract uranium from pitchblende, along with the relevant equations involved in the process.
1. Crushing and Grinding
Pitchblende is first crushed into small pieces and then ground into a fine powder. This step increases the surface area of the ore, allowing for better chemical reactions in subsequent processes.
2. Leaching
Leaching is the process of dissolving the uranium from the powdered pitchblende ore using a suitable chemical solution. The most commonly used leaching agent is sulfuric acid (H2SO4), which reacts with the uranium minerals to form soluble uranium sulfate (UO2SO4).
The chemical equation for leaching uranium from pitchblende can be represented as follows:
U3O8 (pitchblende) + 4H2SO4 (sulfuric acid) → 3UO2SO4 (uranium sulfate) + 4H2O (water)
3. Filtration
After leaching, the resulting solution containing uranium sulfate is separated from the solid residue (gangue) through filtration. Filtration helps remove impurities and other insoluble materials.
4. Purification
The uranium sulfate solution obtained from filtration still contains impurities that need to be removed. One common method for purification is solvent extraction, which involves using an organic solvent to selectively extract uranium from the solution.
5. Precipitation
To obtain solid uranium compounds, the purified uranium solution is further processed through precipitation. Ammonium diuranate (NH4U2O7) is commonly formed by adding ammonium hydroxide (NH4OH) or ammonium carbonate (NH4)2CO3 to the solution. The precipitate is then collected, washed, and dried.
The chemical equation for precipitation of ammonium diuranate can be represented as follows:
UO2SO4 (uranium sulfate) + 2NH4OH (ammonium hydroxide) → NH4U2O7 (ammonium diuranate) + H2SO4 (sulfuric acid) + H2O (water)
6. Conversion to Uranium Oxide
The ammonium diuranate obtained from precipitation is converted into uranium oxide (U3O8) through calcination. Calcination involves heating the ammonium diuranate at high temperatures to remove the remaining impurities and transform it into a more stable oxide form.
The chemical equation for the conversion of ammonium diuranate to uranium oxide can be represented as follows:
2(NH4)2U2O7 (ammonium diuranate) → U3O8 (uranium oxide) + 4NH3 (ammonia) + 3H2O (water)
7. Final Purification
The uranium oxide is further purified using various techniques like solvent extraction, ion exchange, or precipitation to ensure its quality and remove any remaining impurities.
Conclusion
Extracting uranium from pitchblende involves several stages, including crushing and grinding the ore, le
Community Answer
How-to Extract uranium from pitch blende with equation ?

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
How-to Extract uranium from pitch blende with equation ?
Question Description
How-to Extract uranium from pitch blende with equation ? for IIT JAM 2025 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about How-to Extract uranium from pitch blende with equation ? covers all topics & solutions for IIT JAM 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How-to Extract uranium from pitch blende with equation ?.
How-to Extract uranium from pitch blende with equation ? for IIT JAM 2025 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about How-to Extract uranium from pitch blende with equation ? covers all topics & solutions for IIT JAM 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How-to Extract uranium from pitch blende with equation ?.
Solutions for How-to Extract uranium from pitch blende with equation ? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of How-to Extract uranium from pitch blende with equation ? defined & explained in the simplest way possible. Besides giving the explanation of
How-to Extract uranium from pitch blende with equation ?, a detailed solution for How-to Extract uranium from pitch blende with equation ? has been provided alongside types of How-to Extract uranium from pitch blende with equation ? theory, EduRev gives you an
ample number of questions to practice How-to Extract uranium from pitch blende with equation ? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















