Class 12 Exam > Class 12 Questions > The correct statement concerning a SN2 reacti...
Start Learning for Free
The correct statement concerning a SN2 reaction is
- a)the reaction mechanism involve atleast one reactive intermediate
- b)transition state is pentavalent
- c)product is formed after passing through several transition states
- d)nucleophile attacks from front side on which leaving group is present
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The correct statement concerning a SN2 reaction isa)the reaction mecha...
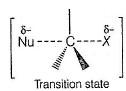
The transition state in SN2 reaction is pentavalent as indicated here.
Most Upvoted Answer
The correct statement concerning a SN2 reaction isa)the reaction mecha...
The Correct Statement Concerning a SN2 Reaction
The correct statement concerning a SN2 (substitution nucleophilic bimolecular) reaction is option B - the transition state is pentavalent.
Explanation:
SN2 reactions are a type of nucleophilic substitution reaction in which a nucleophile replaces a leaving group in a molecule. This reaction involves the simultaneous bond-breaking of the leaving group and the bond-forming of the nucleophile.
In an SN2 reaction, the nucleophile attacks the electrophilic carbon atom (the carbon atom bonded to the leaving group) from the front side, opposite to the leaving group. This is known as front-side attack or backside attack.
Transition State:
During the SN2 reaction, there is a transition state in which the nucleophile is partially bonded to the carbon atom, and the leaving group is partially detached. This transition state is characterized by a pentavalent carbon atom, meaning it has five attached groups. The nucleophile, leaving group, and three other substituents attached to the carbon atom are all present in the transition state.
The pentavalent transition state is formed because the nucleophile starts forming a bond with the carbon atom before the leaving group completely detaches. This transition state is highly unstable and short-lived, and it represents the highest energy point along the reaction pathway.
Reactive Intermediate:
In an SN2 reaction, there is no formation of a reactive intermediate. The reaction occurs in a single step, where the nucleophile directly replaces the leaving group without the formation of any intermediate species.
Product Formation:
In SN2 reactions, the product is formed directly from the transition state without passing through several transition states. Once the nucleophile has fully bonded to the carbon atom, the leaving group completely detaches, and the product is formed.
To summarize, the correct statement concerning an SN2 reaction is that the transition state is pentavalent (option B). This transition state represents the highest energy point in the reaction, and the nucleophile attacks the carbon atom from the front side opposite to the leaving group. The reaction occurs in a single step without the formation of any reactive intermediate, and the product is formed directly from the transition state.
The correct statement concerning a SN2 (substitution nucleophilic bimolecular) reaction is option B - the transition state is pentavalent.
Explanation:
SN2 reactions are a type of nucleophilic substitution reaction in which a nucleophile replaces a leaving group in a molecule. This reaction involves the simultaneous bond-breaking of the leaving group and the bond-forming of the nucleophile.
In an SN2 reaction, the nucleophile attacks the electrophilic carbon atom (the carbon atom bonded to the leaving group) from the front side, opposite to the leaving group. This is known as front-side attack or backside attack.
Transition State:
During the SN2 reaction, there is a transition state in which the nucleophile is partially bonded to the carbon atom, and the leaving group is partially detached. This transition state is characterized by a pentavalent carbon atom, meaning it has five attached groups. The nucleophile, leaving group, and three other substituents attached to the carbon atom are all present in the transition state.
The pentavalent transition state is formed because the nucleophile starts forming a bond with the carbon atom before the leaving group completely detaches. This transition state is highly unstable and short-lived, and it represents the highest energy point along the reaction pathway.
Reactive Intermediate:
In an SN2 reaction, there is no formation of a reactive intermediate. The reaction occurs in a single step, where the nucleophile directly replaces the leaving group without the formation of any intermediate species.
Product Formation:
In SN2 reactions, the product is formed directly from the transition state without passing through several transition states. Once the nucleophile has fully bonded to the carbon atom, the leaving group completely detaches, and the product is formed.
To summarize, the correct statement concerning an SN2 reaction is that the transition state is pentavalent (option B). This transition state represents the highest energy point in the reaction, and the nucleophile attacks the carbon atom from the front side opposite to the leaving group. The reaction occurs in a single step without the formation of any reactive intermediate, and the product is formed directly from the transition state.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer?
Question Description
The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer?.
The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The correct statement concerning a SN2 reaction isa)the reaction mechanism involve atleast one reactive intermediateb)transition state is pentavalentc)product is formed after passing through several transition statesd)nucleophile attacks from front side on which leaving group is presentCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















