Physics Exam > Physics Questions > A Carnot cycle operates on a working substanc...
Start Learning for Free
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?
Select one or more:
Select one or more:
- a)work done in one cycle is Q1 – Q2
- b)entropy for the universe (consisting of the working substance and the two reservoirs) increases
- c)entropy of the hotter reservoir decreases
- d)efficiency is equal to 1
Correct answer is option 'A,B,C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
A Carnot cycle operates on a working substance between two reservoirs ...
Here, the only false statement is efficiency is equal to 1
∴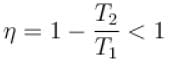
The correct answers are: work done in one cycle is Q1 – Q2, entropy of the hotter reservoir decreases, entropy for the universe (consisting of the working substance and the two reservoirs) increases
∴
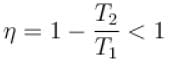
The correct answers are: work done in one cycle is Q1 – Q2, entropy of the hotter reservoir decreases, entropy for the universe (consisting of the working substance and the two reservoirs) increases
Most Upvoted Answer
A Carnot cycle operates on a working substance between two reservoirs ...
Is the higher temperature and T2 is the lower temperature. The Carnot cycle consists of four stages:
1. Isothermal Expansion: The working substance is in contact with the high-temperature reservoir at temperature T1. Heat is added to the working substance, causing it to expand and do work on the surroundings. This stage is isothermal, meaning the temperature remains constant throughout.
2. Adiabatic Expansion: The working substance is now thermally insulated from its surroundings, so no heat can be added or removed. The substance continues to expand, further lowering its temperature.
3. Isothermal Compression: The working substance is now in contact with the low-temperature reservoir at temperature T2. Heat is removed from the substance, causing it to contract and do work on the surroundings. This stage is also isothermal, with the temperature remaining constant.
4. Adiabatic Compression: The working substance is once again thermally insulated, meaning no heat can be added or removed. The substance continues to contract, increasing its temperature.
The Carnot cycle is a theoretical cycle that provides the maximum possible efficiency for a heat engine operating between two temperatures. The efficiency of a Carnot cycle is given by the equation:
Efficiency = 1 - (T2 / T1)
Where T1 is the higher temperature and T2 is the lower temperature. The efficiency of a Carnot cycle can be very high if the temperature difference between the two reservoirs is large.
1. Isothermal Expansion: The working substance is in contact with the high-temperature reservoir at temperature T1. Heat is added to the working substance, causing it to expand and do work on the surroundings. This stage is isothermal, meaning the temperature remains constant throughout.
2. Adiabatic Expansion: The working substance is now thermally insulated from its surroundings, so no heat can be added or removed. The substance continues to expand, further lowering its temperature.
3. Isothermal Compression: The working substance is now in contact with the low-temperature reservoir at temperature T2. Heat is removed from the substance, causing it to contract and do work on the surroundings. This stage is also isothermal, with the temperature remaining constant.
4. Adiabatic Compression: The working substance is once again thermally insulated, meaning no heat can be added or removed. The substance continues to contract, increasing its temperature.
The Carnot cycle is a theoretical cycle that provides the maximum possible efficiency for a heat engine operating between two temperatures. The efficiency of a Carnot cycle is given by the equation:
Efficiency = 1 - (T2 / T1)
Where T1 is the higher temperature and T2 is the lower temperature. The efficiency of a Carnot cycle can be very high if the temperature difference between the two reservoirs is large.
Free Test
FREE
| Start Free Test |
Community Answer
A Carnot cycle operates on a working substance between two reservoirs ...
Only A. and C are the correct answer not B because entropy of the system (carnot cycle)is zero because it Is a reversible process

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer?
Question Description
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer?.
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer?.
Solutions for A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer?, a detailed solution for A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? has been provided alongside types of A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?Select one or more:a)work done in one cycle is Q1 – Q2b)entropy for the universe (consisting of the working substance and the two reservoirs) increasesc)entropy of the hotter reservoir decreasesd)efficiency is equal to 1Correct answer is option 'A,B,C'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















