Physics Exam > Physics Questions > For any process, the second law of thermodyna...
Start Learning for Free
For any process, the second law of thermodynamics requires that the change of entropy of universe is.
Select one:
Select one:
- a)positive only
- b)zero only
- c)positive or zero
- d)negative or zero
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
For any process, the second law of thermodynamics requires that the ch...
From second law thermodynamics, 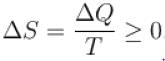
The correct answer is: positive or zero
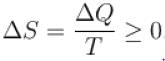
The correct answer is: positive or zero
Most Upvoted Answer
For any process, the second law of thermodynamics requires that the ch...
The Second Law of Thermodynamics and the Change of Entropy of the Universe
The second law of thermodynamics is a fundamental principle in physics that deals with the concept of entropy, which is a measure of the disorder or randomness in a system. According to the second law, the entropy of an isolated system (such as the universe) tends to increase over time.
Entropy and the Change of Entropy
Entropy is a state function that quantifies the number of possible microstates of a system. A microstate refers to the detailed arrangement of particles and energy within a system. The more microstates available to a system, the higher the entropy.
When a process occurs, it can lead to changes in the entropy of the system and its surroundings. The change in entropy is given by the equation:
ΔS = S_final - S_initial
where ΔS represents the change in entropy, S_final is the final entropy, and S_initial is the initial entropy.
The Change of Entropy of the Universe
The universe can be considered as an isolated system, meaning that it does not exchange matter or energy with its surroundings. According to the second law of thermodynamics, the entropy of an isolated system tends to increase. Therefore, for any process occurring in the universe, the change in entropy of the universe (ΔS_universe) will also tend to increase.
Options and Correct Answer
The question presents four options: positive only, zero only, positive or zero, and negative or zero. To determine the correct answer, we need to consider the second law of thermodynamics.
If the change in entropy of the universe is positive only, it means that the entropy of the universe is always increasing, which is in line with the second law. This option is valid.
If the change in entropy of the universe is zero only, it would imply that the entropy of the universe remains constant, which contradicts the second law. This option is invalid.
If the change in entropy of the universe is negative or zero, it would mean that the entropy of the universe either decreases or remains constant, which again contradicts the second law. This option is also invalid.
Therefore, the correct answer is option 'C': positive or zero. This answer aligns with the second law of thermodynamics, which states that the entropy of an isolated system tends to increase.
The second law of thermodynamics is a fundamental principle in physics that deals with the concept of entropy, which is a measure of the disorder or randomness in a system. According to the second law, the entropy of an isolated system (such as the universe) tends to increase over time.
Entropy and the Change of Entropy
Entropy is a state function that quantifies the number of possible microstates of a system. A microstate refers to the detailed arrangement of particles and energy within a system. The more microstates available to a system, the higher the entropy.
When a process occurs, it can lead to changes in the entropy of the system and its surroundings. The change in entropy is given by the equation:
ΔS = S_final - S_initial
where ΔS represents the change in entropy, S_final is the final entropy, and S_initial is the initial entropy.
The Change of Entropy of the Universe
The universe can be considered as an isolated system, meaning that it does not exchange matter or energy with its surroundings. According to the second law of thermodynamics, the entropy of an isolated system tends to increase. Therefore, for any process occurring in the universe, the change in entropy of the universe (ΔS_universe) will also tend to increase.
Options and Correct Answer
The question presents four options: positive only, zero only, positive or zero, and negative or zero. To determine the correct answer, we need to consider the second law of thermodynamics.
If the change in entropy of the universe is positive only, it means that the entropy of the universe is always increasing, which is in line with the second law. This option is valid.
If the change in entropy of the universe is zero only, it would imply that the entropy of the universe remains constant, which contradicts the second law. This option is invalid.
If the change in entropy of the universe is negative or zero, it would mean that the entropy of the universe either decreases or remains constant, which again contradicts the second law. This option is also invalid.
Therefore, the correct answer is option 'C': positive or zero. This answer aligns with the second law of thermodynamics, which states that the entropy of an isolated system tends to increase.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer?
Question Description
For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer?.
For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer?.
Solutions for For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For any process, the second law of thermodynamics requires that the change of entropy of universe is.Select one:a)positive onlyb)zero onlyc)positive or zerod)negative or zeroCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















