NEET Exam > NEET Questions > What is the structure of ETHYLIDENE CHLORIDE ...
Start Learning for Free
What is the structure of ETHYLIDENE CHLORIDE ?
Verified Answer
What is the structure of ETHYLIDENE CHLORIDE ?
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
What is the structure of ETHYLIDENE CHLORIDE ?
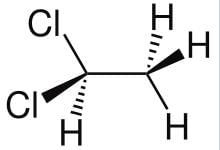
ETHYLIDENE CHLORIDE STRUCTURE:
- Chemical Formula: C2H4Cl2
- Molecular Structure:
- Ethylidene chloride, also known as 1,1-dichloroethane, has a molecular structure with two carbon atoms, four hydrogen atoms, and two chlorine atoms.
- The two chlorine atoms are attached to the same carbon atom, creating a dichloroethane compound.
- Structural Formula:
- The structural formula of ethylidene chloride can be represented as CH3-CHCl2.
- It consists of a central carbon atom bonded to two hydrogen atoms and two chlorine atoms.
- Geometry:
- The geometry of ethylidene chloride is tetrahedral around the carbon atom, with a bond angle of approximately 109.5 degrees.
- The molecule has a bent shape due to the presence of the two chlorine atoms on one side of the central carbon atom.
- Physical Properties:
- Ethylidene chloride is a colorless liquid with a sweet odor.
- It has a boiling point of 57.2 degrees Celsius and a density of 1.245 g/cm3.
- It is slightly soluble in water but miscible with many organic solvents.
- Chemical Properties:
- Ethylidene chloride is primarily used as an intermediate in the production of vinyl chloride, which is used to make PVC.
- It is also used as a solvent in various chemical processes and as a refrigerant.
- Health Hazards:
- Ethylidene chloride is toxic if inhaled, ingested, or absorbed through the skin.
- Prolonged exposure to high concentrations of ethylidene chloride can cause respiratory issues, liver and kidney damage, and central nervous system effects.
- Chemical Formula: C2H4Cl2
- Molecular Structure:
- Ethylidene chloride, also known as 1,1-dichloroethane, has a molecular structure with two carbon atoms, four hydrogen atoms, and two chlorine atoms.
- The two chlorine atoms are attached to the same carbon atom, creating a dichloroethane compound.
- Structural Formula:
- The structural formula of ethylidene chloride can be represented as CH3-CHCl2.
- It consists of a central carbon atom bonded to two hydrogen atoms and two chlorine atoms.
- Geometry:
- The geometry of ethylidene chloride is tetrahedral around the carbon atom, with a bond angle of approximately 109.5 degrees.
- The molecule has a bent shape due to the presence of the two chlorine atoms on one side of the central carbon atom.
- Physical Properties:
- Ethylidene chloride is a colorless liquid with a sweet odor.
- It has a boiling point of 57.2 degrees Celsius and a density of 1.245 g/cm3.
- It is slightly soluble in water but miscible with many organic solvents.
- Chemical Properties:
- Ethylidene chloride is primarily used as an intermediate in the production of vinyl chloride, which is used to make PVC.
- It is also used as a solvent in various chemical processes and as a refrigerant.
- Health Hazards:
- Ethylidene chloride is toxic if inhaled, ingested, or absorbed through the skin.
- Prolonged exposure to high concentrations of ethylidene chloride can cause respiratory issues, liver and kidney damage, and central nervous system effects.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Question Description
What is the structure of ETHYLIDENE CHLORIDE ? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about What is the structure of ETHYLIDENE CHLORIDE ? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the structure of ETHYLIDENE CHLORIDE ?.
What is the structure of ETHYLIDENE CHLORIDE ? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about What is the structure of ETHYLIDENE CHLORIDE ? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the structure of ETHYLIDENE CHLORIDE ?.
Solutions for What is the structure of ETHYLIDENE CHLORIDE ? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of What is the structure of ETHYLIDENE CHLORIDE ? defined & explained in the simplest way possible. Besides giving the explanation of
What is the structure of ETHYLIDENE CHLORIDE ?, a detailed solution for What is the structure of ETHYLIDENE CHLORIDE ? has been provided alongside types of What is the structure of ETHYLIDENE CHLORIDE ? theory, EduRev gives you an
ample number of questions to practice What is the structure of ETHYLIDENE CHLORIDE ? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























