JEE Exam > JEE Questions > 1-phenyl ethanol SOCl2 gives [x] Compound x ...
Start Learning for Free
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how?
Most Upvoted Answer
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) reten...
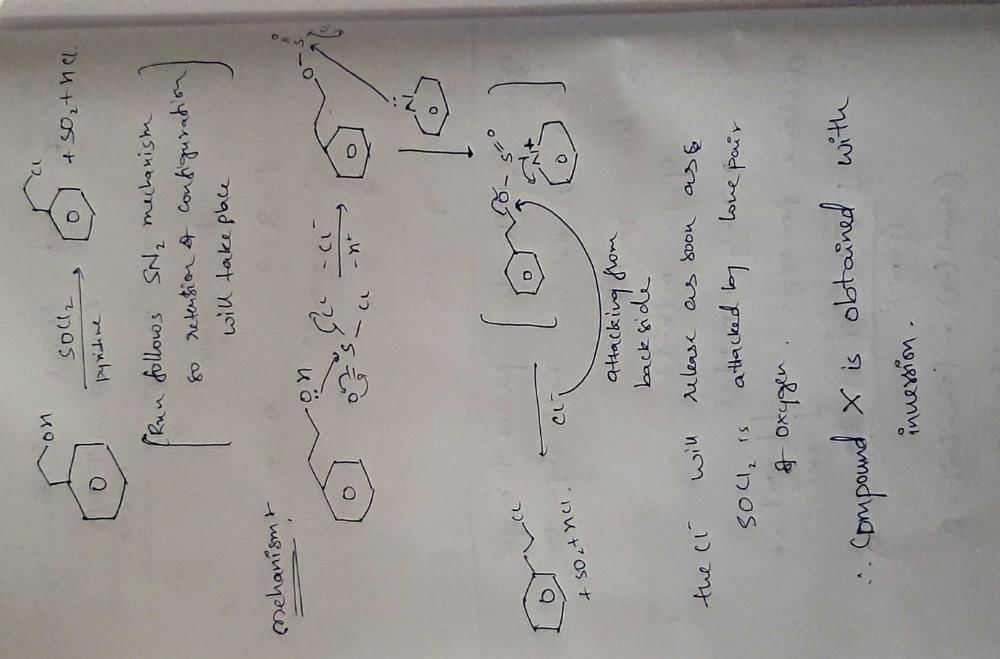
Community Answer
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) reten...
Retention of Configuration in SN2 Reaction of 1-Phenyl Ethanol with SOCl2
The reaction of 1-phenyl ethanol (C6H5CH(OH)CH3) with thionyl chloride (SOCl2) leads to the formation of a new compound, denoted as [x]. The correct option is i) retention of configuration, which means that the stereochemistry of the starting material is preserved in the product.
In order to understand why the reaction proceeds with retention of configuration, let's analyze the reaction mechanism.
1. Reaction Mechanism:
The reaction between 1-phenyl ethanol and SOCl2 proceeds via an SN2 (substitution nucleophilic bimolecular) mechanism. In this mechanism, the nucleophile (Cl-) attacks the carbon atom, resulting in the displacement of the leaving group (OH-) and the formation of a new bond.
2. Key Factors:
a) Steric Hindrance: The presence of a bulky phenyl group attached to the carbon atom bearing the hydroxyl group (OH-) creates steric hindrance. This hindrance restricts the approach of the nucleophile from the back side, favoring an SN2 mechanism.
b) Inversion vs. Retention: In an SN2 reaction, the nucleophile attacks the carbon atom from the back side, leading to inversion of configuration if the substrate is a chiral compound. However, in the case of 1-phenyl ethanol, the phenyl group prevents complete inversion of configuration.
3. Explanation:
Due to the steric hindrance caused by the phenyl group, the nucleophile (Cl-) cannot approach the carbon atom carrying the hydroxyl group (OH-) from the back side. Instead, it approaches from the front side, resulting in the formation of the new bond while maintaining the same configuration as the starting material.
This phenomenon is known as retention of configuration. The bulky phenyl group acts as a protective shield, preventing the complete inversion of configuration that would normally occur in an SN2 reaction.
Therefore, the correct option for the compound [x] obtained from the reaction of 1-phenyl ethanol with SOCl2 is i) retention of configuration.
In conclusion, the steric hindrance caused by the phenyl group in 1-phenyl ethanol allows the reaction with SOCl2 to proceed via an SN2 mechanism with retention of configuration.
The reaction of 1-phenyl ethanol (C6H5CH(OH)CH3) with thionyl chloride (SOCl2) leads to the formation of a new compound, denoted as [x]. The correct option is i) retention of configuration, which means that the stereochemistry of the starting material is preserved in the product.
In order to understand why the reaction proceeds with retention of configuration, let's analyze the reaction mechanism.
1. Reaction Mechanism:
The reaction between 1-phenyl ethanol and SOCl2 proceeds via an SN2 (substitution nucleophilic bimolecular) mechanism. In this mechanism, the nucleophile (Cl-) attacks the carbon atom, resulting in the displacement of the leaving group (OH-) and the formation of a new bond.
2. Key Factors:
a) Steric Hindrance: The presence of a bulky phenyl group attached to the carbon atom bearing the hydroxyl group (OH-) creates steric hindrance. This hindrance restricts the approach of the nucleophile from the back side, favoring an SN2 mechanism.
b) Inversion vs. Retention: In an SN2 reaction, the nucleophile attacks the carbon atom from the back side, leading to inversion of configuration if the substrate is a chiral compound. However, in the case of 1-phenyl ethanol, the phenyl group prevents complete inversion of configuration.
3. Explanation:
Due to the steric hindrance caused by the phenyl group, the nucleophile (Cl-) cannot approach the carbon atom carrying the hydroxyl group (OH-) from the back side. Instead, it approaches from the front side, resulting in the formation of the new bond while maintaining the same configuration as the starting material.
This phenomenon is known as retention of configuration. The bulky phenyl group acts as a protective shield, preventing the complete inversion of configuration that would normally occur in an SN2 reaction.
Therefore, the correct option for the compound [x] obtained from the reaction of 1-phenyl ethanol with SOCl2 is i) retention of configuration.
In conclusion, the steric hindrance caused by the phenyl group in 1-phenyl ethanol allows the reaction with SOCl2 to proceed via an SN2 mechanism with retention of configuration.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how?
Question Description
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how?.
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how?.
Solutions for 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? defined & explained in the simplest way possible. Besides giving the explanation of
1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how?, a detailed solution for 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? has been provided alongside types of 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? theory, EduRev gives you an
ample number of questions to practice 1-phenyl ethanol SOCl2 gives [x] Compound x obtained is with i) retention ii) inversion iii) racemic mixture Correct option is i) retention Can u explain this how? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























