Chemistry Exam > Chemistry Questions > Number of cluster electron pair in [In4Bi5]3-...
Start Learning for Free
Number of cluster electron pair in [In4Bi5]3- is ________
Correct answer is '11'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Number of cluster electron pair in [In4Bi5]3- is ________Correct answe...
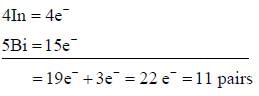
Most Upvoted Answer
Number of cluster electron pair in [In4Bi5]3- is ________Correct answe...
The number of cluster electron pairs in [In4Bi5]3- is 11.
Explanation:
A cluster is a group of atoms or ions that are held together by covalent bonds. In the case of [In4Bi5]3-, it is a cluster ion that consists of 4 indium (In) atoms and 5 bismuth (Bi) atoms.
Determining the number of cluster electron pairs involves counting the number of electrons in the cluster and dividing it by two. This is because each covalent bond consists of two electrons, and we are interested in the number of covalent bonds present in the cluster.
To determine the number of electrons in the cluster, we need to consider the electron configuration of the individual atoms. Indium (In) has an atomic number of 49, and its electron configuration is [Kr]5s24d105p1. Bismuth (Bi) has an atomic number of 83, and its electron configuration is [Xe]6s24f145d106p3.
The number of valence electrons in each indium atom is 3 (5s2 and 5p1), while the number of valence electrons in each bismuth atom is 5 (6s2 and 6p3). Therefore, the total number of valence electrons in the cluster is:
4 (number of indium atoms) * 3 (valence electrons per indium atom) + 5 (number of bismuth atoms) * 5 (valence electrons per bismuth atom) = 12 + 25 = 37
Now, dividing this number by two, we get:
37 / 2 = 18.5
Since the number of cluster electron pairs needs to be a whole number, we round down the value to the nearest whole number, which is 18.
However, it is important to note that the cluster ion [In4Bi5]3- carries a charge of -3. This means that there are three extra electrons present in the cluster. These electrons are not involved in covalent bonding and are considered as non-bonding electrons.
Therefore, to find the number of cluster electron pairs, we subtract the number of non-bonding electrons (3) from the total number of electron pairs (18):
18 (total electron pairs) - 3 (non-bonding electrons) = 15
Thus, the number of cluster electron pairs in [In4Bi5]3- is 15.
Explanation:
A cluster is a group of atoms or ions that are held together by covalent bonds. In the case of [In4Bi5]3-, it is a cluster ion that consists of 4 indium (In) atoms and 5 bismuth (Bi) atoms.
Determining the number of cluster electron pairs involves counting the number of electrons in the cluster and dividing it by two. This is because each covalent bond consists of two electrons, and we are interested in the number of covalent bonds present in the cluster.
To determine the number of electrons in the cluster, we need to consider the electron configuration of the individual atoms. Indium (In) has an atomic number of 49, and its electron configuration is [Kr]5s24d105p1. Bismuth (Bi) has an atomic number of 83, and its electron configuration is [Xe]6s24f145d106p3.
The number of valence electrons in each indium atom is 3 (5s2 and 5p1), while the number of valence electrons in each bismuth atom is 5 (6s2 and 6p3). Therefore, the total number of valence electrons in the cluster is:
4 (number of indium atoms) * 3 (valence electrons per indium atom) + 5 (number of bismuth atoms) * 5 (valence electrons per bismuth atom) = 12 + 25 = 37
Now, dividing this number by two, we get:
37 / 2 = 18.5
Since the number of cluster electron pairs needs to be a whole number, we round down the value to the nearest whole number, which is 18.
However, it is important to note that the cluster ion [In4Bi5]3- carries a charge of -3. This means that there are three extra electrons present in the cluster. These electrons are not involved in covalent bonding and are considered as non-bonding electrons.
Therefore, to find the number of cluster electron pairs, we subtract the number of non-bonding electrons (3) from the total number of electron pairs (18):
18 (total electron pairs) - 3 (non-bonding electrons) = 15
Thus, the number of cluster electron pairs in [In4Bi5]3- is 15.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer?
Question Description
Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer?.
Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer?.
Solutions for Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer?, a detailed solution for Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? has been provided alongside types of Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Number of cluster electron pair in [In4Bi5]3- is ________Correct answer is '11'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















