Chemistry Exam > Chemistry Questions > The most stable oxidation state of Uranium is...
Start Learning for Free
The most stable oxidation state of Uranium is ........
Correct answer is '6'. Can you explain this answer?
Verified Answer
The most stable oxidation state of Uranium is ........Correct answer i...
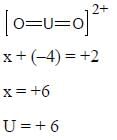
Most Upvoted Answer
The most stable oxidation state of Uranium is ........Correct answer i...
The Most Stable Oxidation State of Uranium is +6
Uranium is a radioactive element with atomic number 92 and symbol U. It belongs to the actinide series of elements in the periodic table. Uranium has multiple oxidation states, ranging from +3 to +6, with +6 being the most stable oxidation state.
Explanation:
1. Electronic Configuration:
The electronic configuration of uranium is [Rn]5f^36d^17s^2. In its ground state, uranium has seven valence electrons in the 5f and 6d orbitals.
2. Stability of Oxidation States:
The stability of an oxidation state depends on various factors such as the electronic configuration, ionization energy, and the ability of the element to gain or lose electrons.
3. Ionization Energy:
The ionization energy of uranium increases as we move from lower to higher oxidation states. This means that it requires more energy to remove an electron from uranium in higher oxidation states.
4. Stability of +6 Oxidation State:
The +6 oxidation state of uranium is the most stable because it has a completely filled 5f and 6d orbitals. This configuration is more stable due to the presence of the half-filled 5f orbital, which provides extra stability.
5. Formation of Uranium Hexafluoride (UF6):
One of the common compounds formed by uranium in its +6 oxidation state is uranium hexafluoride (UF6). UF6 is a volatile compound used in the enrichment of uranium for nuclear reactors and weapons. The formation of UF6 is energetically favorable, contributing to the stability of the +6 oxidation state.
6. Other Oxidation States:
Although the +6 oxidation state is the most stable, uranium can also exist in other oxidation states such as +3, +4, and +5. However, these oxidation states are relatively less stable and are less commonly observed in compounds.
In conclusion, the most stable oxidation state of uranium is +6. This state is favored due to the complete filling of the 5f and 6d orbitals, as well as the formation of energetically favorable compounds like uranium hexafluoride (UF6).
Uranium is a radioactive element with atomic number 92 and symbol U. It belongs to the actinide series of elements in the periodic table. Uranium has multiple oxidation states, ranging from +3 to +6, with +6 being the most stable oxidation state.
Explanation:
1. Electronic Configuration:
The electronic configuration of uranium is [Rn]5f^36d^17s^2. In its ground state, uranium has seven valence electrons in the 5f and 6d orbitals.
2. Stability of Oxidation States:
The stability of an oxidation state depends on various factors such as the electronic configuration, ionization energy, and the ability of the element to gain or lose electrons.
3. Ionization Energy:
The ionization energy of uranium increases as we move from lower to higher oxidation states. This means that it requires more energy to remove an electron from uranium in higher oxidation states.
4. Stability of +6 Oxidation State:
The +6 oxidation state of uranium is the most stable because it has a completely filled 5f and 6d orbitals. This configuration is more stable due to the presence of the half-filled 5f orbital, which provides extra stability.
5. Formation of Uranium Hexafluoride (UF6):
One of the common compounds formed by uranium in its +6 oxidation state is uranium hexafluoride (UF6). UF6 is a volatile compound used in the enrichment of uranium for nuclear reactors and weapons. The formation of UF6 is energetically favorable, contributing to the stability of the +6 oxidation state.
6. Other Oxidation States:
Although the +6 oxidation state is the most stable, uranium can also exist in other oxidation states such as +3, +4, and +5. However, these oxidation states are relatively less stable and are less commonly observed in compounds.
In conclusion, the most stable oxidation state of uranium is +6. This state is favored due to the complete filling of the 5f and 6d orbitals, as well as the formation of energetically favorable compounds like uranium hexafluoride (UF6).

|
Explore Courses for Chemistry exam
|

|
Question Description
The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer?.
The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer?.
Solutions for The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer?, a detailed solution for The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? has been provided alongside types of The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The most stable oxidation state of Uranium is ........Correct answer is '6'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























