NEET Exam > NEET Questions > Kolbe electrolysis ?
Start Learning for Free
Kolbe electrolysis ?
Verified Answer
Kolbe electrolysis ?
Kolbe reaction" redirects here. For the carboxylation of phenols, see Kolbe–Schmitt reaction.
The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe.[1][2] The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions) The overall general reaction is:
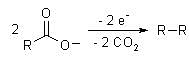
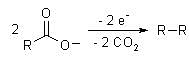
If a mixture of two different carboxylates are used, all combinations of them are generally seen as the organic product structures:
3 R1COO− + 3 R2COO− → R1−R1 + R1−R2 + R2−R2 + 6 CO2 + 6 e−
The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate, then two such intermediates combine to form a covalent bond.[3] As an example, electrolysis of acetic acid yields ethane and carbon dioxide:
CH3COOH → CH3COO− → CH3COO� → CH3� + CO2
2CH3� → CH3CH3
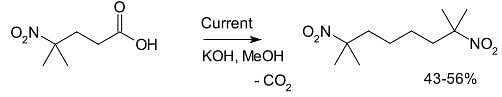
Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid:[4]
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
Kolbe electrolysis ?
Kolbe Electrolysis: An Overview
Kolbe electrolysis, also known as the Kolbe reaction, is an important organic transformation that occurs during the electrolysis of certain carboxylic acids or their salts. This process involves the decarboxylation of carboxylic acids and the subsequent formation of alkane derivatives.
The Kolbe Electrolysis Mechanism:
The Kolbe electrolysis mechanism involves the following steps:
1. Anodic Oxidation: The carboxylic acid or its salt is subjected to electrolysis in an electrolytic cell. The anode (positive electrode) serves as the site of oxidation. Here, the carboxylate anion is oxidized to a radical cation.
2. Formation of a Radical Intermediate: The radical cation formed at the anode undergoes decarboxylation, resulting in the formation of a radical intermediate. This radical intermediate is highly reactive and can participate in various reactions.
3. Coupling Reaction: The radical intermediate formed in the previous step then undergoes a coupling reaction with another radical intermediate. This reaction involves the combination of two radicals to form a covalent bond. The coupling reaction leads to the formation of a dimer product.
4. Reduction of the Radical Dimer: The dimer product formed in the previous step is subsequently reduced. This reduction occurs at the cathode (negative electrode) of the electrolytic cell. The reduction process involves the gain of electrons by the dimer product, leading to the formation of an alkane derivative.
Applications of Kolbe Electrolysis:
Kolbe electrolysis has several practical applications in organic chemistry:
1. Synthesis of Alkanes: The primary application of Kolbe electrolysis is the synthesis of alkanes. By subjecting carboxylic acids or their salts to electrolysis, it is possible to convert them into alkane derivatives. This process offers an alternative method for the preparation of alkanes, especially those with complex structures.
2. Formation of Carbon-Carbon Bonds: Kolbe electrolysis plays a significant role in the formation of carbon-carbon bonds. The coupling reaction during the Kolbe electrolysis mechanism allows for the creation of new carbon-carbon bonds, enabling the synthesis of more complex organic compounds.
3. Production of Intermediates: The radical intermediates formed during Kolbe electrolysis can serve as important intermediates in various organic reactions. These intermediates can be further transformed into desired organic compounds, thereby expanding the synthetic possibilities in organic chemistry.
In conclusion, Kolbe electrolysis is a valuable method for the synthesis of alkanes and the formation of carbon-carbon bonds. By understanding the mechanism and applications of this reaction, organic chemists can utilize Kolbe electrolysis to access a wide range of organic compounds.
Kolbe electrolysis, also known as the Kolbe reaction, is an important organic transformation that occurs during the electrolysis of certain carboxylic acids or their salts. This process involves the decarboxylation of carboxylic acids and the subsequent formation of alkane derivatives.
The Kolbe Electrolysis Mechanism:
The Kolbe electrolysis mechanism involves the following steps:
1. Anodic Oxidation: The carboxylic acid or its salt is subjected to electrolysis in an electrolytic cell. The anode (positive electrode) serves as the site of oxidation. Here, the carboxylate anion is oxidized to a radical cation.
2. Formation of a Radical Intermediate: The radical cation formed at the anode undergoes decarboxylation, resulting in the formation of a radical intermediate. This radical intermediate is highly reactive and can participate in various reactions.
3. Coupling Reaction: The radical intermediate formed in the previous step then undergoes a coupling reaction with another radical intermediate. This reaction involves the combination of two radicals to form a covalent bond. The coupling reaction leads to the formation of a dimer product.
4. Reduction of the Radical Dimer: The dimer product formed in the previous step is subsequently reduced. This reduction occurs at the cathode (negative electrode) of the electrolytic cell. The reduction process involves the gain of electrons by the dimer product, leading to the formation of an alkane derivative.
Applications of Kolbe Electrolysis:
Kolbe electrolysis has several practical applications in organic chemistry:
1. Synthesis of Alkanes: The primary application of Kolbe electrolysis is the synthesis of alkanes. By subjecting carboxylic acids or their salts to electrolysis, it is possible to convert them into alkane derivatives. This process offers an alternative method for the preparation of alkanes, especially those with complex structures.
2. Formation of Carbon-Carbon Bonds: Kolbe electrolysis plays a significant role in the formation of carbon-carbon bonds. The coupling reaction during the Kolbe electrolysis mechanism allows for the creation of new carbon-carbon bonds, enabling the synthesis of more complex organic compounds.
3. Production of Intermediates: The radical intermediates formed during Kolbe electrolysis can serve as important intermediates in various organic reactions. These intermediates can be further transformed into desired organic compounds, thereby expanding the synthetic possibilities in organic chemistry.
In conclusion, Kolbe electrolysis is a valuable method for the synthesis of alkanes and the formation of carbon-carbon bonds. By understanding the mechanism and applications of this reaction, organic chemists can utilize Kolbe electrolysis to access a wide range of organic compounds.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Kolbe electrolysis ?
Question Description
Kolbe electrolysis ? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Kolbe electrolysis ? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Kolbe electrolysis ?.
Kolbe electrolysis ? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Kolbe electrolysis ? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Kolbe electrolysis ?.
Solutions for Kolbe electrolysis ? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Kolbe electrolysis ? defined & explained in the simplest way possible. Besides giving the explanation of
Kolbe electrolysis ?, a detailed solution for Kolbe electrolysis ? has been provided alongside types of Kolbe electrolysis ? theory, EduRev gives you an
ample number of questions to practice Kolbe electrolysis ? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























