Class 10 Exam > Class 10 Questions > Silver articles become black on prolonged exp...
Start Learning for Free
Silver articles become black on prolonged exposure to air. This is due to the formation of
- a)Ag3N
- b)Ag2O
- c)Ag2S
- d)Ag2S and Ag3N
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Silver articles become black on prolonged exposure to air. This is due...
When the silver reacts with the hydrogen sulfide gas that is present in the air to form a black coating of silver sulfide. Silver sulfide is insoluble in all the solvents.
The balanced chemical reaction will be,
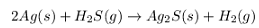
By stoichiometry, 2 moles of silver react with the 1 mole of hydrogen gas to give 1 mole of silver sulfide and 2 moles of hydrogen gas.
You can learn all the concepts of
You can learn all the concepts of
Chemical Reactions and Equations by going through the chapter:
Most Upvoted Answer
Silver articles become black on prolonged exposure to air. This is due...
Option (c) becoz--->
Silver articles when exposed to air become black after sometime. This is because the silver metal reacts with sulphur present in the atmosphere and forms silver sulphide (black colour).
Ag (s) + H2S (g) → Ag2S (s) + H2 (g)
Silver articles when exposed to air become black after sometime. This is because the silver metal reacts with sulphur present in the atmosphere and forms silver sulphide (black colour).
Ag (s) + H2S (g) → Ag2S (s) + H2 (g)
Free Test
FREE
| Start Free Test |
Community Answer
Silver articles become black on prolonged exposure to air. This is due...
Why do silver articles become black on prolonged exposure to air?
The blackening of silver articles on prolonged exposure to air is due to the formation of a layer of silver sulfide (Ag2S) on the surface of the silver. This is a chemical reaction that occurs between the silver and sulfur compounds in the air.
Explanation:
- Chemical Reaction: The reaction between silver and sulfur compounds in the air is a chemical reaction that results in the formation of silver sulfide. The reaction can be represented by the following equation: 2Ag + H2S + O2 → Ag2S + H2O
- Formation of Silver Sulfide: The silver sulfide that forms on the surface of the silver is a black compound that gives the silver article its black appearance. Silver sulfide is insoluble in water, which means it cannot be easily removed from the surface of the silver.
- Air Pollution: The reaction between silver and sulfur compounds in the air is accelerated by air pollution. Sulfur dioxide (SO2) is a common air pollutant that can react with silver to form silver sulfide. This is why silver articles tend to blacken more quickly in areas with high levels of air pollution.
- Prevention: The blackening of silver articles can be prevented by storing them in airtight containers or by using anti-tarnish strips. These strips contain chemicals that react with sulfur compounds in the air, preventing them from reacting with the silver. Regular cleaning and polishing can also help to prevent the formation of silver sulfide on the surface of silver articles.
Conclusion:
In conclusion, the blackening of silver articles on prolonged exposure to air is due to the formation of a layer of silver sulfide on the surface of the silver. This is a chemical reaction that occurs between the silver and sulfur compounds in the air. The reaction can be prevented by storing silver articles in airtight containers, using anti-tarnish strips, and regular cleaning and polishing.
The blackening of silver articles on prolonged exposure to air is due to the formation of a layer of silver sulfide (Ag2S) on the surface of the silver. This is a chemical reaction that occurs between the silver and sulfur compounds in the air.
Explanation:
- Chemical Reaction: The reaction between silver and sulfur compounds in the air is a chemical reaction that results in the formation of silver sulfide. The reaction can be represented by the following equation: 2Ag + H2S + O2 → Ag2S + H2O
- Formation of Silver Sulfide: The silver sulfide that forms on the surface of the silver is a black compound that gives the silver article its black appearance. Silver sulfide is insoluble in water, which means it cannot be easily removed from the surface of the silver.
- Air Pollution: The reaction between silver and sulfur compounds in the air is accelerated by air pollution. Sulfur dioxide (SO2) is a common air pollutant that can react with silver to form silver sulfide. This is why silver articles tend to blacken more quickly in areas with high levels of air pollution.
- Prevention: The blackening of silver articles can be prevented by storing them in airtight containers or by using anti-tarnish strips. These strips contain chemicals that react with sulfur compounds in the air, preventing them from reacting with the silver. Regular cleaning and polishing can also help to prevent the formation of silver sulfide on the surface of silver articles.
Conclusion:
In conclusion, the blackening of silver articles on prolonged exposure to air is due to the formation of a layer of silver sulfide on the surface of the silver. This is a chemical reaction that occurs between the silver and sulfur compounds in the air. The reaction can be prevented by storing silver articles in airtight containers, using anti-tarnish strips, and regular cleaning and polishing.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer?
Question Description
Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer?.
Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Silver articles become black on prolonged exposure to air. This is due to the formation ofa)Ag3Nb)Ag2Oc)Ag2Sd)Ag2S and Ag3NCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.