JEE Exam > JEE Questions > With reference to aqua regia, choose the corr...
Start Learning for Free
With reference to aqua regia, choose the correct option(s).
- a)Reaction of gold with aqua regia produces NO2 in the absence of air
- b)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratio
- c)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation state
- d)The yellow colour of aqua regia is due to the presence of NOCl and Cl2
Correct answer is option 'B,C,D'. Can you explain this answer?
Verified Answer
With reference to aqua regia, choose the correct option(s).a)Reaction ...
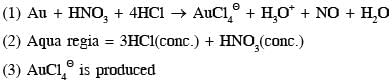
(4) Yellow colour of aqua regia is due to it's decomposition into NOCl(orange yellow) and Cl2(greenish yellow).
Most Upvoted Answer
With reference to aqua regia, choose the correct option(s).a)Reaction ...
Aqua Regia
Aqua regia is a mixture of concentrated hydrochloric acid and concentrated nitric acid in a 3:1 (v/v) ratio. It is a highly corrosive and strong oxidizing agent that is used to dissolve noble metals such as gold and platinum.
Reactions of Gold with Aqua Regia
When gold is reacted with aqua regia, the following reactions take place:
- Au + 3HCl + 4HNO3 → Au(NO3)3 + 3NO2 + 3H2O + 3Cl
- Au + HNO3 + 4HCl → AuCl4^- + NO + 2H2O
From these reactions, we can conclude the following:
- Reaction of gold with aqua regia produces NO2 gas in the absence of air.
- The anion produced during the reaction of gold with aqua regia has Au in the 3 oxidation state.
- The yellow color of aqua regia is due to the presence of NO2 and Cl2 gases.
Conclusion
In conclusion, aqua regia is a powerful and highly corrosive mixture of concentrated hydrochloric acid and concentrated nitric acid. It is used to dissolve noble metals such as gold and platinum. When gold is reacted with aqua regia, it produces NO2 gas, an anion with Au in the 3 oxidation state, and gives aqua regia its yellow color due to the presence of NO2 and Cl2 gases.
Aqua regia is a mixture of concentrated hydrochloric acid and concentrated nitric acid in a 3:1 (v/v) ratio. It is a highly corrosive and strong oxidizing agent that is used to dissolve noble metals such as gold and platinum.
Reactions of Gold with Aqua Regia
When gold is reacted with aqua regia, the following reactions take place:
- Au + 3HCl + 4HNO3 → Au(NO3)3 + 3NO2 + 3H2O + 3Cl
- Au + HNO3 + 4HCl → AuCl4^- + NO + 2H2O
From these reactions, we can conclude the following:
- Reaction of gold with aqua regia produces NO2 gas in the absence of air.
- The anion produced during the reaction of gold with aqua regia has Au in the 3 oxidation state.
- The yellow color of aqua regia is due to the presence of NO2 and Cl2 gases.
Conclusion
In conclusion, aqua regia is a powerful and highly corrosive mixture of concentrated hydrochloric acid and concentrated nitric acid. It is used to dissolve noble metals such as gold and platinum. When gold is reacted with aqua regia, it produces NO2 gas, an anion with Au in the 3 oxidation state, and gives aqua regia its yellow color due to the presence of NO2 and Cl2 gases.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer?
Question Description
With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer?.
With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer?.
Solutions for With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer?, a detailed solution for With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? has been provided alongside types of With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice With reference to aqua regia, choose the correct option(s).a)Reaction of gold with aqua regia produces NO2 in the absence of airb)Aqua regia is prepared by mixing conc. HCl and conc. HNO3 in 3 : 1 (v/v) ratioc)Reaction of gold with aqua regia produces an anion having Au in +3 oxidation stated)The yellow colour of aqua regia is due to the presence of NOCl and Cl2Correct answer is option 'B,C,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























