Physics Exam > Physics Questions > Packing fraction of diamond cubic crystal str...
Start Learning for Free
Packing fraction of diamond cubic crystal structure is ____ % ?
Correct answer is '34'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Packing fraction of diamond cubic crystal structure is ____ % ?Correct...
Number of atoms in diamond crystal
Packing fraction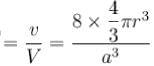
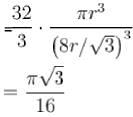
= 0.34
= 34%
The correct answer is: 34
Packing fraction
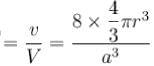
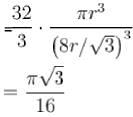
= 0.34
= 34%
The correct answer is: 34
Most Upvoted Answer
Packing fraction of diamond cubic crystal structure is ____ % ?Correct...
Packing Fraction of Diamond Cubic Structure
The packing fraction (also known as packing efficiency) is a measure of how much of a material's volume is occupied by its constituent particles (atoms, in this case). For the diamond cubic crystal structure, the packing fraction is approximately 34%.
Understanding Diamond Cubic Structure
- The diamond cubic structure consists of two interpenetrating face-centered cubic (FCC) lattices.
- Each unit cell contains 8 atoms: 4 from one FCC lattice and 4 from another.
Calculation of Packing Fraction
- Volume of Atoms: In a diamond cubic structure, each atom can be approximated as a sphere. The volume of a single atom (considered a sphere) is calculated using the formula for the volume of a sphere.
- Unit Cell Volume: The volume of the cubic unit cell is determined by the cube of the edge length (a^3).
Packing Fraction Formula
The packing fraction is calculated using the formula:
Packing Fraction = (Total Volume of Atoms in Unit Cell) / (Volume of Unit Cell)
- The total volume occupied by the atoms in the unit cell is derived from their arrangement and the number of atoms present.
- For diamond cubic, the maximum packing fraction comes out to be around 34% after performing the necessary calculations.
Significance of 34%
- A packing fraction of 34% indicates that a significant portion of the volume in a diamond cubic structure is empty space.
- This low packing efficiency is characteristic of the diamond structure, contributing to its unique properties such as hardness and low density compared to metals.
In conclusion, the diamond cubic crystal structure's packing fraction of 34% reflects the efficient arrangement and bonding of carbon atoms, leading to the material's notable physical properties.
The packing fraction (also known as packing efficiency) is a measure of how much of a material's volume is occupied by its constituent particles (atoms, in this case). For the diamond cubic crystal structure, the packing fraction is approximately 34%.
Understanding Diamond Cubic Structure
- The diamond cubic structure consists of two interpenetrating face-centered cubic (FCC) lattices.
- Each unit cell contains 8 atoms: 4 from one FCC lattice and 4 from another.
Calculation of Packing Fraction
- Volume of Atoms: In a diamond cubic structure, each atom can be approximated as a sphere. The volume of a single atom (considered a sphere) is calculated using the formula for the volume of a sphere.
- Unit Cell Volume: The volume of the cubic unit cell is determined by the cube of the edge length (a^3).
Packing Fraction Formula
The packing fraction is calculated using the formula:
Packing Fraction = (Total Volume of Atoms in Unit Cell) / (Volume of Unit Cell)
- The total volume occupied by the atoms in the unit cell is derived from their arrangement and the number of atoms present.
- For diamond cubic, the maximum packing fraction comes out to be around 34% after performing the necessary calculations.
Significance of 34%
- A packing fraction of 34% indicates that a significant portion of the volume in a diamond cubic structure is empty space.
- This low packing efficiency is characteristic of the diamond structure, contributing to its unique properties such as hardness and low density compared to metals.
In conclusion, the diamond cubic crystal structure's packing fraction of 34% reflects the efficient arrangement and bonding of carbon atoms, leading to the material's notable physical properties.
Free Test
FREE
| Start Free Test |
Community Answer
Packing fraction of diamond cubic crystal structure is ____ % ?Correct...
Number of atoms in diamond crystal
Packing fraction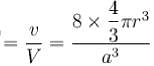
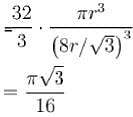
= 0.34
= 34%
The correct answer is: 34
Packing fraction
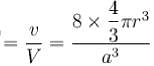
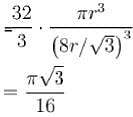
= 0.34
= 34%
The correct answer is: 34

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer?
Question Description
Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer?.
Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? for Physics 2024 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? covers all topics & solutions for Physics 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer?.
Solutions for Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer?, a detailed solution for Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? has been provided alongside types of Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Packing fraction of diamond cubic crystal structure is ____ % ?Correct answer is '34'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















