UPSC Exam > UPSC Questions > Isotopes have the same atomic number but dif...
Start Learning for Free
Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?
1. Treatment of cancer
2. Fuel in nuclear reactors
3. Treatment of goitre
Select the correct answer using the code given below.
- a)1 and 2 only
- b)1 and 3 only
- c)2 and 3 only
- d)1, 2 and 3
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Isotopes have the same atomic number but different atomic masses. Whi...
Hydrogen has three isotopes i,e protium, deutorium and tritium,,,
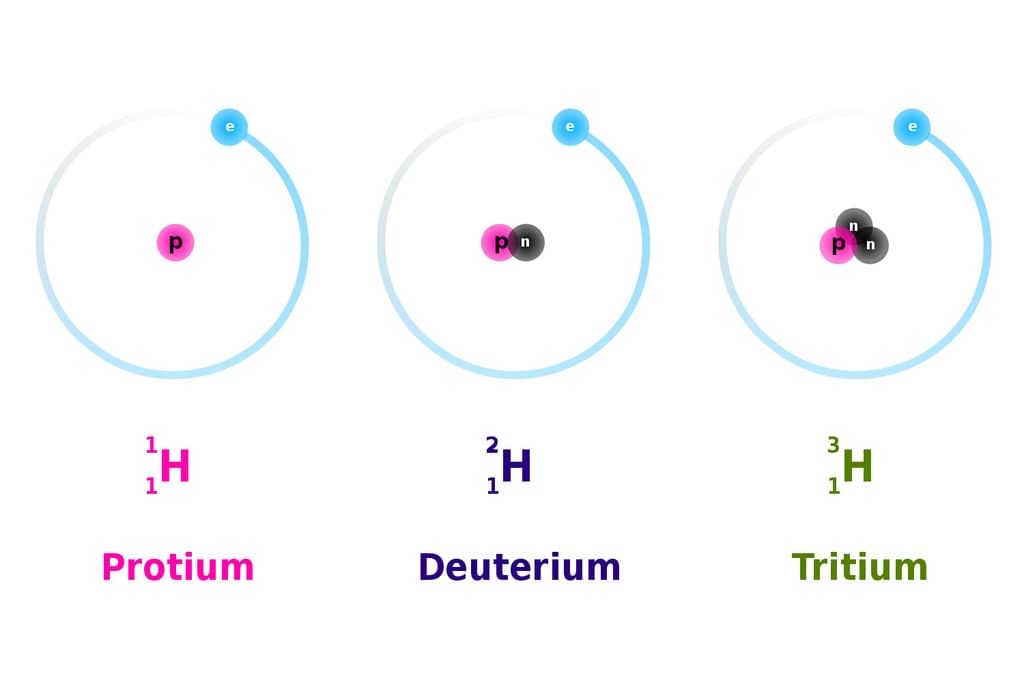
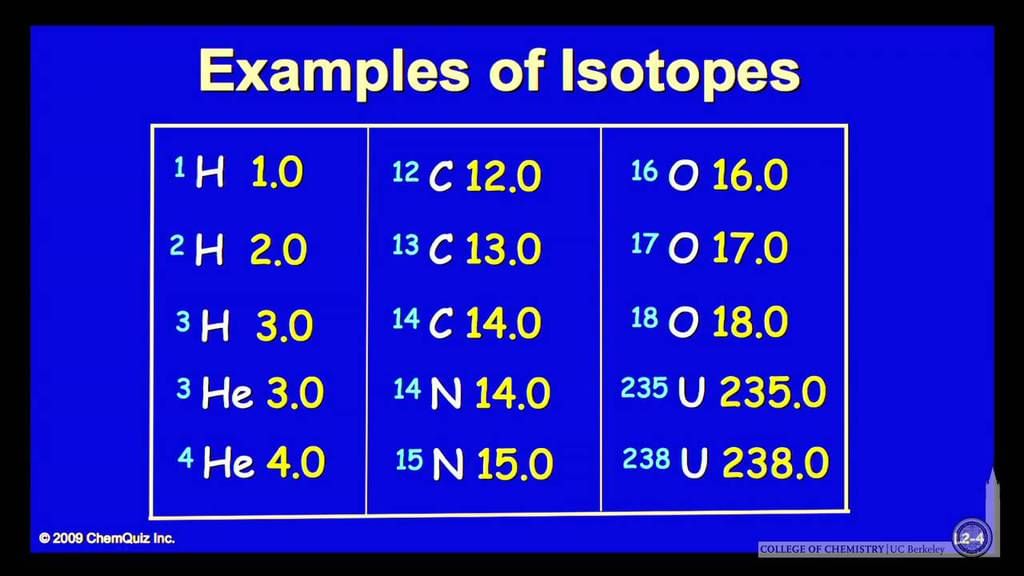
some different examples are, helium, carbon, nitrogen, uranium


Free Test
FREE
| Start Free Test |
Community Answer
Isotopes have the same atomic number but different atomic masses. Whi...
The correct answer is option 'D' - 1, 2 and 3.
Radioactive isotopes are isotopes of an element that have an unstable nucleus and emit radiation in the form of particles or electromagnetic waves. These isotopes have various uses in different fields. Let's discuss each of the given uses in detail:
1. Treatment of cancer:
Radioactive isotopes are widely used in the treatment of cancer. They can be used to target and kill cancer cells. One such example is the use of radioactive isotopes in brachytherapy. In this treatment, small radioactive sources are placed near or inside the tumor. These sources emit radiation that damages the DNA of cancer cells, preventing them from growing and dividing. This helps in shrinking or eliminating the tumor.
2. Fuel in nuclear reactors:
Radioactive isotopes, such as uranium-235 and plutonium-239, are used as fuel in nuclear reactors. These isotopes undergo nuclear fission, a process in which the nucleus of an atom splits into two or more smaller nuclei, releasing a large amount of energy. This energy is utilized to generate electricity in nuclear power plants. The controlled fission of radioactive isotopes provides a reliable and efficient source of energy.
3. Treatment of goitre:
Radioactive iodine isotopes, specifically iodine-131, are used in the treatment of goitre, a condition characterized by the enlargement of the thyroid gland. Iodine-131 emits radiation that can selectively destroy the overactive thyroid tissue, reducing the size of the gland and treating the symptoms associated with goitre. This treatment is known as radioiodine therapy.
In conclusion, radioactive isotopes have various important uses. They are used in the treatment of cancer, as fuel in nuclear reactors, and in the treatment of goitre. These applications highlight the significant role of radioactive isotopes in medicine, energy production, and healthcare.
Radioactive isotopes are isotopes of an element that have an unstable nucleus and emit radiation in the form of particles or electromagnetic waves. These isotopes have various uses in different fields. Let's discuss each of the given uses in detail:
1. Treatment of cancer:
Radioactive isotopes are widely used in the treatment of cancer. They can be used to target and kill cancer cells. One such example is the use of radioactive isotopes in brachytherapy. In this treatment, small radioactive sources are placed near or inside the tumor. These sources emit radiation that damages the DNA of cancer cells, preventing them from growing and dividing. This helps in shrinking or eliminating the tumor.
2. Fuel in nuclear reactors:
Radioactive isotopes, such as uranium-235 and plutonium-239, are used as fuel in nuclear reactors. These isotopes undergo nuclear fission, a process in which the nucleus of an atom splits into two or more smaller nuclei, releasing a large amount of energy. This energy is utilized to generate electricity in nuclear power plants. The controlled fission of radioactive isotopes provides a reliable and efficient source of energy.
3. Treatment of goitre:
Radioactive iodine isotopes, specifically iodine-131, are used in the treatment of goitre, a condition characterized by the enlargement of the thyroid gland. Iodine-131 emits radiation that can selectively destroy the overactive thyroid tissue, reducing the size of the gland and treating the symptoms associated with goitre. This treatment is known as radioiodine therapy.
In conclusion, radioactive isotopes have various important uses. They are used in the treatment of cancer, as fuel in nuclear reactors, and in the treatment of goitre. These applications highlight the significant role of radioactive isotopes in medicine, energy production, and healthcare.
Attention UPSC Students!
To make sure you are not studying endlessly, EduRev has designed UPSC study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in UPSC.

|
Explore Courses for UPSC exam
|

|
Similar UPSC Doubts
Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer?
Question Description
Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? for UPSC 2024 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UPSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer?.
Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? for UPSC 2024 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for UPSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer?.
Solutions for Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for UPSC.
Download more important topics, notes, lectures and mock test series for UPSC Exam by signing up for free.
Here you can find the meaning of Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Isotopes have the same atomic number but different atomic masses. Which among the following are the uses of radioactive isotopes?1. Treatment of cancer2. Fuel in nuclear reactors3. Treatment of goitreSelect the correct answer using the code given below.a) 1 and 2 onlyb) 1 and 3 onlyc) 2 and 3 onlyd) 1, 2 and 3Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice UPSC tests.

|
Explore Courses for UPSC exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























