Mechanical Engineering Exam > Mechanical Engineering Questions > A rigid tank of volume of 8 m3 is being fille...
Start Learning for Free
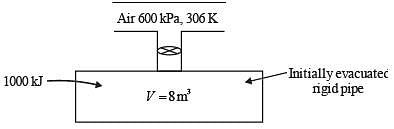
A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.
The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).
The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).
Correct answer is '395.39'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
A rigid tank of volume of 8 m3 is being filled up with air from a pipe...
Unsteady flow energy equation :
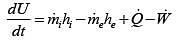
∵ (me = 0 and W = 0)
We Know
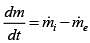
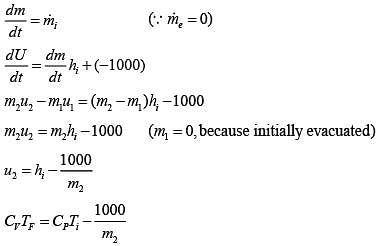
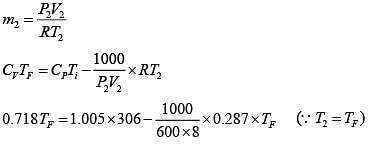
TF = 395.389 K
 395.39 K
395.39 KHence, the final temperature of the tank after the completion of the filling process is 395.39 K.
Free Test
FREE
| Start Free Test |
Community Answer
A rigid tank of volume of 8 m3 is being filled up with air from a pipe...
Given data:
- Volume of the tank (V) = 8 m^3
- Initial pressure inside the pipeline (P1) = 600 kPa
- Initial temperature inside the pipeline (T1) = 306 K
- Heat loss to the surroundings (Q) = 1000 kJ
- Specific heat at constant pressure (Cp) = 1.005 kJ/kg.K
- Specific heat at constant volume (Cv) = 0.718 kJ/kg.K
To find:
- Final temperature of the tank (T2)
Assumptions:
- The process is quasi-static (slow) and reversible.
- There is no work done during the process.
- Neglect changes in kinetic energy and potential energy.
Solution:
The process of filling the tank can be considered as an isochoric process (constant volume) because the volume of the tank remains constant.
Step 1: Determining the mass of air in the tank
Using the ideal gas equation, the mass of air in the tank can be calculated as:
m = P1*V / (R*T1)
where R is the specific gas constant for air (287 J/kg.K).
Step 2: Determining the heat added to the air
The heat added to the air during the filling process can be calculated using the first law of thermodynamics:
Q = m*Cv*(T2 - T1)
Rearranging the equation, we get:
T2 = (Q / (m*Cv)) + T1
Step 3: Substituting the given values
Substituting the given values into the equations:
m = (600,000 Pa * 8 m^3) / (287 J/kg.K * 306 K)
m = 168.9 kg
T2 = (1000 kJ / (168.9 kg * 0.718 kJ/kg.K)) + 306 K
T2 = 395.39 K
Therefore, the final temperature of the tank after the completion of the filling process is approximately 395.39 K (rounded off to the nearest integer as 395 K).
- Volume of the tank (V) = 8 m^3
- Initial pressure inside the pipeline (P1) = 600 kPa
- Initial temperature inside the pipeline (T1) = 306 K
- Heat loss to the surroundings (Q) = 1000 kJ
- Specific heat at constant pressure (Cp) = 1.005 kJ/kg.K
- Specific heat at constant volume (Cv) = 0.718 kJ/kg.K
To find:
- Final temperature of the tank (T2)
Assumptions:
- The process is quasi-static (slow) and reversible.
- There is no work done during the process.
- Neglect changes in kinetic energy and potential energy.
Solution:
The process of filling the tank can be considered as an isochoric process (constant volume) because the volume of the tank remains constant.
Step 1: Determining the mass of air in the tank
Using the ideal gas equation, the mass of air in the tank can be calculated as:
m = P1*V / (R*T1)
where R is the specific gas constant for air (287 J/kg.K).
Step 2: Determining the heat added to the air
The heat added to the air during the filling process can be calculated using the first law of thermodynamics:
Q = m*Cv*(T2 - T1)
Rearranging the equation, we get:
T2 = (Q / (m*Cv)) + T1
Step 3: Substituting the given values
Substituting the given values into the equations:
m = (600,000 Pa * 8 m^3) / (287 J/kg.K * 306 K)
m = 168.9 kg
T2 = (1000 kJ / (168.9 kg * 0.718 kJ/kg.K)) + 306 K
T2 = 395.39 K
Therefore, the final temperature of the tank after the completion of the filling process is approximately 395.39 K (rounded off to the nearest integer as 395 K).
Attention Mechanical Engineering Students!
To make sure you are not studying endlessly, EduRev has designed Mechanical Engineering study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Mechanical Engineering.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer?
Question Description
A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer?.
A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer?.
Solutions for A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer?, a detailed solution for A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? has been provided alongside types of A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg.K and 0.718 kJ/kg.K, respectively. Neglect changes in kinetic energy and potential energy.The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer).Correct answer is '395.39'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























