CTET & State TET Exam > CTET & State TET Questions > How many times be a solution of pH = 3 dilute...
Start Learning for Free
How many times be a solution of pH = 3 diluted to get a solution of pH = 6?
- a)2
- b)10
- c)100
- d)1000
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
How many times be a solution of pH = 3 diluted to get a solution of pH...
For pH = 3, [H+] = 10-3
For pH = 6, the [H+] required = 10-6
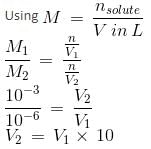
Therefore, the sample has to be diluted 103 times.
For pH = 6, the [H+] required = 10-6
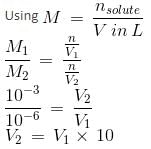
Therefore, the sample has to be diluted 103 times.
Most Upvoted Answer
How many times be a solution of pH = 3 diluted to get a solution of pH...
For pH = 3, [H+] = 10-3
For pH = 6, the [H+] required = 10-6
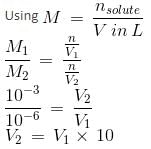
Therefore, the sample has to be diluted 103 times.
For pH = 6, the [H+] required = 10-6
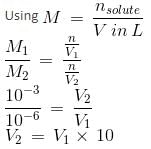
Therefore, the sample has to be diluted 103 times.
Free Test
FREE
| Start Free Test |
Community Answer
How many times be a solution of pH = 3 diluted to get a solution of pH...
To understand why the correct answer is option 'D', let's break down the problem step by step.
1. pH Scale:
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, where 0 is highly acidic, 7 is neutral, and 14 is highly alkaline. Each unit on the pH scale represents a tenfold difference in acidity or alkalinity.
2. pH and Hydrogen Ion Concentration:
pH is calculated based on the concentration of hydrogen ions (H+) in a solution. The lower the pH, the higher the concentration of H+ ions, and the more acidic the solution. The higher the pH, the lower the concentration of H+ ions, and the more alkaline the solution.
3. Dilution and pH:
When a solution is diluted, the concentration of H+ ions decreases. This means that the pH of the solution will increase. Dilution has the effect of reducing the acidity of a solution.
4. Relationship between pH Values:
To determine how many times a solution of pH 3 must be diluted to reach a pH of 6, we need to consider the difference in hydrogen ion concentration between the two solutions.
- pH 3 has a hydrogen ion concentration of 10^(-3) moles per liter (M).
- pH 6 has a hydrogen ion concentration of 10^(-6) M.
To reach a pH of 6 from pH 3, the concentration of H+ ions needs to decrease by a factor of 10^3. This means that the solution needs to be diluted 1000 times.
Therefore, the correct answer is option 'D' - 1000.
1. pH Scale:
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, where 0 is highly acidic, 7 is neutral, and 14 is highly alkaline. Each unit on the pH scale represents a tenfold difference in acidity or alkalinity.
2. pH and Hydrogen Ion Concentration:
pH is calculated based on the concentration of hydrogen ions (H+) in a solution. The lower the pH, the higher the concentration of H+ ions, and the more acidic the solution. The higher the pH, the lower the concentration of H+ ions, and the more alkaline the solution.
3. Dilution and pH:
When a solution is diluted, the concentration of H+ ions decreases. This means that the pH of the solution will increase. Dilution has the effect of reducing the acidity of a solution.
4. Relationship between pH Values:
To determine how many times a solution of pH 3 must be diluted to reach a pH of 6, we need to consider the difference in hydrogen ion concentration between the two solutions.
- pH 3 has a hydrogen ion concentration of 10^(-3) moles per liter (M).
- pH 6 has a hydrogen ion concentration of 10^(-6) M.
To reach a pH of 6 from pH 3, the concentration of H+ ions needs to decrease by a factor of 10^3. This means that the solution needs to be diluted 1000 times.
Therefore, the correct answer is option 'D' - 1000.

|
Explore Courses for CTET & State TET exam
|

|
Question Description
How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? for CTET & State TET 2025 is part of CTET & State TET preparation. The Question and answers have been prepared according to the CTET & State TET exam syllabus. Information about How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for CTET & State TET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer?.
How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? for CTET & State TET 2025 is part of CTET & State TET preparation. The Question and answers have been prepared according to the CTET & State TET exam syllabus. Information about How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for CTET & State TET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer?.
Solutions for How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for CTET & State TET.
Download more important topics, notes, lectures and mock test series for CTET & State TET Exam by signing up for free.
Here you can find the meaning of How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer?, a detailed solution for How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice How many times be a solution of pH = 3 diluted to get a solution of pH = 6?a)2b)10c)100d)1000Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice CTET & State TET tests.

|
Explore Courses for CTET & State TET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.