UGC NET Exam > UGC NET Questions > For a transition metal M, the correct order o...
Start Learning for Free
For a transition metal M, the correct order of 13C NMR spectral shift [in ppm relative to [Si(CH3)4] for the moieties M-CH3, M-CO and M-C6H5, is
- a)M-CH3 < M-C6H5 < M-CO
- b)M-CO < M-CH3 < M-C6H5
- c)M-C6H5 < M-CH3 < M-CO
- d)M-CO < M-C6H5 < M-CH3
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
For a transition metal M, the correct order of13C NMR spectral shift [...
13C NMR:
- The Carbon-13 nuclear magnetic resonance or 13C NMR spectroscopy is the application of nuclear magnetic resonance spectroscopy to carbon (C). 13C NMR spectroscopy helps to identify the nature or environment of C atoms in organic molecules just as 1H NMR spectroscopy helps to detect the H atoms.
- The nature or environment of C atoms in organic molecules can be identified by chemical shift (δ) values.
- The chemical shift values of some common functional groups are as follows:
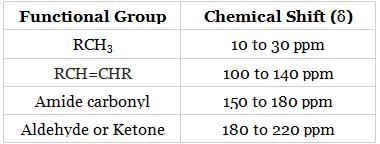
- From the above table, we can conclude that, the chemical shift value will be highest for M-CO (180 to 220 ppm), followed by M-C6H5 (100 to 140 ppm) and M-CH3 (10 to 30 ppm).
- Thus, the correct order of 13C NMR spectral shift [in ppm relative to [Si(CH3)4] for the moieties M-CH3, M-CO and M-C6H5, is M-CH3 < M-C6H5 < M-CO
Hence, for a transition metal M, the correct order of 13C NMR spectral shift [in ppm relative to [Si(CH3)4] for the moieties M-CH3, M-CO, and M-C6H5, is M-CH3 < M-C6H5 < M-CO
Most Upvoted Answer
For a transition metal M, the correct order of13C NMR spectral shift [...
Explanation:
Chemical Shift in 13C NMR Spectra:
- The chemical shift in 13C NMR spectra is influenced by the electronic environment surrounding the carbon atom being observed.
- Different functional groups or ligands attached to a transition metal M will result in distinct shifts in chemical shift values.
Order of Spectral Shift:
- The correct order of 13C NMR spectral shift relative to [Si(CH3)4] for the moieties M-CH3, M-CO, and M-C6H5 is as follows:
a) M-CH3 < M-C6H5 < M-CO
- This order indicates that the M-CH3 group will have the least chemical shift compared to M-C6H5 and M-CO in the 13C NMR spectra.
- The M-CO group would exhibit the highest chemical shift among the three moieties.
- This order can be attributed to the electronic effects of the different ligands on the transition metal M.
- The M-CH3 group, being alkyl in nature, will have lower electron density compared to M-C6H5 (aromatic) and M-CO (carbonyl), leading to a lower chemical shift.
- Therefore, the correct order of spectral shift reflects the electronic environment and the impact of different ligands on the chemical shift values in 13C NMR spectra.
Chemical Shift in 13C NMR Spectra:
- The chemical shift in 13C NMR spectra is influenced by the electronic environment surrounding the carbon atom being observed.
- Different functional groups or ligands attached to a transition metal M will result in distinct shifts in chemical shift values.
Order of Spectral Shift:
- The correct order of 13C NMR spectral shift relative to [Si(CH3)4] for the moieties M-CH3, M-CO, and M-C6H5 is as follows:
a) M-CH3 < M-C6H5 < M-CO
- This order indicates that the M-CH3 group will have the least chemical shift compared to M-C6H5 and M-CO in the 13C NMR spectra.
- The M-CO group would exhibit the highest chemical shift among the three moieties.
- This order can be attributed to the electronic effects of the different ligands on the transition metal M.
- The M-CH3 group, being alkyl in nature, will have lower electron density compared to M-C6H5 (aromatic) and M-CO (carbonyl), leading to a lower chemical shift.
- Therefore, the correct order of spectral shift reflects the electronic environment and the impact of different ligands on the chemical shift values in 13C NMR spectra.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer?
Question Description
For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer?.
For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer?.
Solutions for For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer?, a detailed solution for For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For a transition metal M, the correct order of13C NMR spectral shift [in ppm relativeto[Si(CH3)4] for the moietiesM-CH3, M-CO and M-C6H5, isa)M-CH3< M-C6H5<M-COb)M-CO < M-CH3< M-C6H5c)M-C6H5< M-CH3<M-COd)M-CO < M-C6H5 < M-CH3Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























