UGC NET Exam > UGC NET Questions > In the vibrational spectrum of CO2, the numbe...
Start Learning for Free
In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman are
- a)Two
- b)three
- c)zero
- d)one
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
In the vibrational spectrum of CO2, the number of fundamental vibratio...
- CO2 is a linear molecule.
- The rotation of a linear molecule changes with the angle between the molecule and the 2 perpendicular axes.
- CO2 which is a linear molecule has two rotational degrees of freedom.
- Thus, for linear molecules like CO2, the vibrational degree of freedom is 4.
- CO2 molecule that possesses a centre of symmetry.
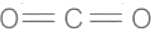
- Thus, in the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman are Zero. (According to Rule of mutual exclusion)
Hence, option 3 is the correct answer.
Most Upvoted Answer
In the vibrational spectrum of CO2, the number of fundamental vibratio...
CO2 is a linear molecule with three atoms - one carbon atom in the center and two oxygen atoms on either side. In the vibrational spectrum of CO2, the different vibrational modes can be categorized into two types: infrared (IR) active modes and Raman active modes.
IR Active Modes:
IR active modes are vibrations that result in a change in the dipole moment of the molecule and can be observed in the infrared spectrum. For a linear molecule like CO2, the number of IR active modes can be determined by applying the selection rule Δμ ≠ 0, where Δμ is the change in the dipole moment.
Raman Active Modes:
Raman active modes are vibrations that result in a change in polarizability of the molecule and can be observed in the Raman spectrum. The number of Raman active modes for a linear molecule can be determined by applying the selection rule Δα ≠ 0, where Δα is the change in polarizability.
Number of Fundamental Vibrational Modes:
In the case of CO2, each oxygen atom has a double bond with the central carbon atom. This results in four vibrational modes - two stretching modes and two bending modes.
Stretching Modes:
The stretching modes involve the vibrations of the two oxygen atoms away from the central carbon atom. There are two stretching modes in CO2 - one in which both oxygen atoms move towards or away from the carbon atom simultaneously (symmetric stretching), and the other in which the oxygen atoms move in opposite directions (antisymmetric stretching).
Bending Modes:
The bending modes involve the vibrations of the oxygen atoms towards or away from the central carbon atom. There are two bending modes in CO2 - one in which both oxygen atoms move towards or away from the carbon atom simultaneously (symmetric bending), and the other in which the oxygen atoms move in opposite directions (antisymmetric bending).
Since CO2 is a linear molecule, the symmetric stretching and symmetric bending modes do not result in a change in dipole moment or polarizability. Therefore, these modes are not IR or Raman active. Only the antisymmetric stretching mode results in a change in dipole moment and polarizability, making it both IR and Raman active.
Hence, in the vibrational spectrum of CO2, there is only one fundamental vibrational mode that is common to both the infrared and Raman spectra. Thus, the correct answer is option 'C' - zero.
IR Active Modes:
IR active modes are vibrations that result in a change in the dipole moment of the molecule and can be observed in the infrared spectrum. For a linear molecule like CO2, the number of IR active modes can be determined by applying the selection rule Δμ ≠ 0, where Δμ is the change in the dipole moment.
Raman Active Modes:
Raman active modes are vibrations that result in a change in polarizability of the molecule and can be observed in the Raman spectrum. The number of Raman active modes for a linear molecule can be determined by applying the selection rule Δα ≠ 0, where Δα is the change in polarizability.
Number of Fundamental Vibrational Modes:
In the case of CO2, each oxygen atom has a double bond with the central carbon atom. This results in four vibrational modes - two stretching modes and two bending modes.
Stretching Modes:
The stretching modes involve the vibrations of the two oxygen atoms away from the central carbon atom. There are two stretching modes in CO2 - one in which both oxygen atoms move towards or away from the carbon atom simultaneously (symmetric stretching), and the other in which the oxygen atoms move in opposite directions (antisymmetric stretching).
Bending Modes:
The bending modes involve the vibrations of the oxygen atoms towards or away from the central carbon atom. There are two bending modes in CO2 - one in which both oxygen atoms move towards or away from the carbon atom simultaneously (symmetric bending), and the other in which the oxygen atoms move in opposite directions (antisymmetric bending).
Since CO2 is a linear molecule, the symmetric stretching and symmetric bending modes do not result in a change in dipole moment or polarizability. Therefore, these modes are not IR or Raman active. Only the antisymmetric stretching mode results in a change in dipole moment and polarizability, making it both IR and Raman active.
Hence, in the vibrational spectrum of CO2, there is only one fundamental vibrational mode that is common to both the infrared and Raman spectra. Thus, the correct answer is option 'C' - zero.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer?
Question Description
In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer?.
In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer?.
Solutions for In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In the vibrational spectrum of CO2, the number of fundamental vibrational modes common in both infrared and Raman area)Twob)threec)zerod)oneCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























