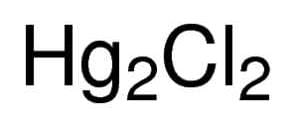Class 10 Exam > Class 10 Questions > chemical formula of mercury
Start Learning for Free
chemical formula of mercury
Most Upvoted Answer
chemical formula of mercury
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪˈdrɑːrdʒərəm/ hy-DRAR-jər-əm).[4] A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury is a heavy, silvery-white liquid metal. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity.[5]
It has a freezing point of −38.83 °C and a boiling point of 356.73 °C,[6][7][8] both the lowest of any stable metal, although preliminary experiments on copernicium and flerovium have indicated that they have even lower boiling points (copernicium being the element below mercury in the periodic table, following the trend of decreasing boiling points down group 12).[9] Upon freezing, the volume of mercury decreases by 3.59% and its density changes from 13.69 g/cm3 when liquid to 14.184 g/cm3 when solid. The coefficient of volume expansion is 181.59 × 10−6 at 0 °C, 181.71 × 10−6 at 20 °C and 182.50 × 10−6 at 100 °C (per °C). Solid mercury is malleable and ductile and can be cut with a knife.[10]
A complete explanation of mercury's extreme volatility delves deep into the realm of quantum physics, but it can be summarized as follows: mercury has a unique electron configuration where electrons fill up all the available 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, and 6s subshells. Because this configuration strongly resists removal of an electron, mercury behaves similarly to noble gases, which form weak bonds and hence melt at low temperatures.
The stability of the 6s shell is due to the presence of a filled 4f shell. An f shell poorly screens the nuclear charge that increases the attractive Coulomb interaction of the 6s shell and the nucleus (see lanthanide contraction). The absence of a filled inner f shell is the reason for the somewhat higher melting temperature of cadmium and zinc, although both these metals still melt easily and, in addition, have unusually low boiling points.[6][7]
Chemical properties
Mercury does not react with most acids, such as dilute sulfuric acid, although oxidizing acids such as concentrated sulfuric acid and nitric acid or aqua regia dissolve it to give sulfate, nitrate, and chloride. Like silver, mercury reacts with atmospheric hydrogen sulfide. Mercury reacts with solid sulfur flakes, which are used in mercury spill kits to absorb mercury (spill kits also use activated carbon and powdered zinc).[11]
Mercury is a heavy, silvery-white liquid metal. Compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity.[5]
It has a freezing point of −38.83 °C and a boiling point of 356.73 °C,[6][7][8] both the lowest of any stable metal, although preliminary experiments on copernicium and flerovium have indicated that they have even lower boiling points (copernicium being the element below mercury in the periodic table, following the trend of decreasing boiling points down group 12).[9] Upon freezing, the volume of mercury decreases by 3.59% and its density changes from 13.69 g/cm3 when liquid to 14.184 g/cm3 when solid. The coefficient of volume expansion is 181.59 × 10−6 at 0 °C, 181.71 × 10−6 at 20 °C and 182.50 × 10−6 at 100 °C (per °C). Solid mercury is malleable and ductile and can be cut with a knife.[10]
A complete explanation of mercury's extreme volatility delves deep into the realm of quantum physics, but it can be summarized as follows: mercury has a unique electron configuration where electrons fill up all the available 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, and 6s subshells. Because this configuration strongly resists removal of an electron, mercury behaves similarly to noble gases, which form weak bonds and hence melt at low temperatures.
The stability of the 6s shell is due to the presence of a filled 4f shell. An f shell poorly screens the nuclear charge that increases the attractive Coulomb interaction of the 6s shell and the nucleus (see lanthanide contraction). The absence of a filled inner f shell is the reason for the somewhat higher melting temperature of cadmium and zinc, although both these metals still melt easily and, in addition, have unusually low boiling points.[6][7]
Chemical properties
Mercury does not react with most acids, such as dilute sulfuric acid, although oxidizing acids such as concentrated sulfuric acid and nitric acid or aqua regia dissolve it to give sulfate, nitrate, and chloride. Like silver, mercury reacts with atmospheric hydrogen sulfide. Mercury reacts with solid sulfur flakes, which are used in mercury spill kits to absorb mercury (spill kits also use activated carbon and powdered zinc).[11]
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
chemical formula of mercury
Question Description
chemical formula of mercury for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about chemical formula of mercury covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for chemical formula of mercury .
chemical formula of mercury for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about chemical formula of mercury covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for chemical formula of mercury .
Solutions for chemical formula of mercury in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of chemical formula of mercury defined & explained in the simplest way possible. Besides giving the explanation of
chemical formula of mercury , a detailed solution for chemical formula of mercury has been provided alongside types of chemical formula of mercury theory, EduRev gives you an
ample number of questions to practice chemical formula of mercury tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.