Chemistry Exam > Chemistry Questions > Molecular shape of SOCl2 isa)Square planarb)T...
Start Learning for Free
Molecular shape of SOCl2 is
- a)Square planar
- b)Trigonal pyramidal
- c)Triangular planar
- d)T-shape
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triang...
SOCl2 has a trigonal pyramidal shape. This molecule is of the AX E3 type and has a tetrahedral electron-group geometry and a trigonal pyramidal shape.
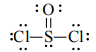
Most Upvoted Answer
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triang...
Molecular shape of SOCl2 is Trigonal pyramidal.
Explanation:
SOCl2 is the chemical formula for thionyl chloride. It consists of one sulfur atom (S), one oxygen atom (O), and two chlorine atoms (Cl). To determine the molecular shape of SOCl2, we need to consider the arrangement of the atoms around the central sulfur atom.
- Central atom: Sulfur (S)
- Surrounding atoms: Oxygen (O) and two Chlorine (Cl) atoms
1. Lewis structure:
First, let's draw the Lewis structure of SOCl2 to determine the arrangement of the valence electrons. The Lewis structure shows the bonding and non-bonding electron pairs around the central atom.
- Sulfur (S) has 6 valence electrons.
- Oxygen (O) has 6 valence electrons.
- Chlorine (Cl) has 7 valence electrons each.
When we combine these atoms, we get:
O Cl Cl
\ | /
S
To complete the octet of sulfur, one of the oxygen atoms forms a double bond with sulfur, and the other two atoms (one oxygen and one chlorine) form single bonds. This leaves one lone pair of electrons on the sulfur atom.
2. Electron pair geometry:
To determine the electron pair geometry, we need to consider both the bonded and lone pair electrons around the central atom.
In SOCl2, we have:
- 1 double bond (2 electron pairs) between S and O
- 2 single bonds (2 electron pairs) between S and Cl
- 1 lone pair (2 electron pairs) on S
So, the total number of electron pairs around the central atom is 6.
3. Molecular shape:
The molecular shape is determined by the arrangement of these electron pairs. In this case, the electron pair geometry is trigonal bipyramidal.
However, we need to consider the positions of the bonded and lone pairs in this geometry to determine the actual molecular shape.
In SOCl2, we have:
- 1 bonded pair (double bond) and 3 bonded pairs (single bonds)
- 1 lone pair
The presence of a lone pair causes repulsion, which results in the distortion of the electron pair geometry. The lone pair occupies more space than the bonded pairs, pushing the bonded pairs closer together.
As a result, the molecular shape of SOCl2 is trigonal pyramidal, where the three chlorine atoms and the lone pair are positioned in a pyramid-like arrangement around the central sulfur atom.
Therefore, the correct answer is option B: Trigonal pyramidal.
Explanation:
SOCl2 is the chemical formula for thionyl chloride. It consists of one sulfur atom (S), one oxygen atom (O), and two chlorine atoms (Cl). To determine the molecular shape of SOCl2, we need to consider the arrangement of the atoms around the central sulfur atom.
- Central atom: Sulfur (S)
- Surrounding atoms: Oxygen (O) and two Chlorine (Cl) atoms
1. Lewis structure:
First, let's draw the Lewis structure of SOCl2 to determine the arrangement of the valence electrons. The Lewis structure shows the bonding and non-bonding electron pairs around the central atom.
- Sulfur (S) has 6 valence electrons.
- Oxygen (O) has 6 valence electrons.
- Chlorine (Cl) has 7 valence electrons each.
When we combine these atoms, we get:
O Cl Cl
\ | /
S
To complete the octet of sulfur, one of the oxygen atoms forms a double bond with sulfur, and the other two atoms (one oxygen and one chlorine) form single bonds. This leaves one lone pair of electrons on the sulfur atom.
2. Electron pair geometry:
To determine the electron pair geometry, we need to consider both the bonded and lone pair electrons around the central atom.
In SOCl2, we have:
- 1 double bond (2 electron pairs) between S and O
- 2 single bonds (2 electron pairs) between S and Cl
- 1 lone pair (2 electron pairs) on S
So, the total number of electron pairs around the central atom is 6.
3. Molecular shape:
The molecular shape is determined by the arrangement of these electron pairs. In this case, the electron pair geometry is trigonal bipyramidal.
However, we need to consider the positions of the bonded and lone pairs in this geometry to determine the actual molecular shape.
In SOCl2, we have:
- 1 bonded pair (double bond) and 3 bonded pairs (single bonds)
- 1 lone pair
The presence of a lone pair causes repulsion, which results in the distortion of the electron pair geometry. The lone pair occupies more space than the bonded pairs, pushing the bonded pairs closer together.
As a result, the molecular shape of SOCl2 is trigonal pyramidal, where the three chlorine atoms and the lone pair are positioned in a pyramid-like arrangement around the central sulfur atom.
Therefore, the correct answer is option B: Trigonal pyramidal.
Free Test
FREE
| Start Free Test |
Community Answer
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triang...
According to VSEPR theory Sulphur contain 1 lone pair and 3 sigma bond then geometry is trigonal pyramidal.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer?
Question Description
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer?.
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Molecular shape of SOCl2 isa)Square planarb)Trigonal pyramidalc)Triangular planard)T-shapeCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















