Class 10 Exam > Class 10 Questions > Properties of covalent compound?
Start Learning for Free
Properties of covalent compound?
Verified Answer
Properties of covalent compound?
(i) Physical State : Covalent compounds can exist in solid, liquid as well as gaseous state e.g., CH4 is gas, CHCl3 is liquid, glucose is solid.
(ii) Solubility :
(a) They are generally insoluble in water and in polar solvents because they cannot form ions in aqueous solution.
(b) They are soluble in non-polar organic solvents like ether, benzene, CCl4, CS2, CHCl3, acetone etc.
(iii) Electrical Conductivity : Covalent compounds are poor conductors of electricity because they do not contain ions or free electrons for conduction of electricity, e.g., CCl4, benzene, toluene do not conduct electricity.
(iv) Melting and Boiling Point : Melting and boiling points of covalent compounds are low due to weak forces of attraction between molecules. Less energy is required to overcome these forces of attraction, e.g.,
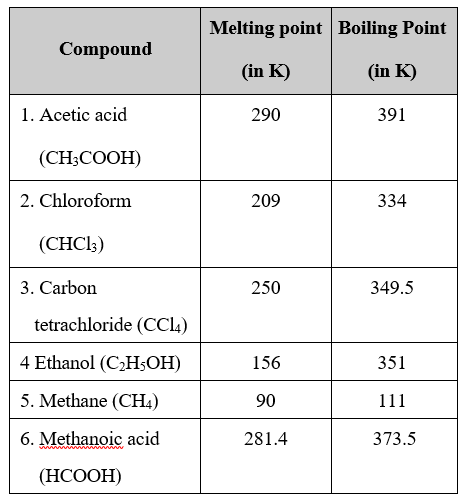

 This question is part of UPSC exam. View all Class 10 courses
This question is part of UPSC exam. View all Class 10 courses
Most Upvoted Answer
Properties of covalent compound?
1.Most covalent compounds have relatively low melting points and boiling points
2.Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds
3.Covalent compounds tend to be more flammable than ionic compounds.
4.When dissolved in water, they don'tconduct electricity.
2.Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds
3.Covalent compounds tend to be more flammable than ionic compounds.
4.When dissolved in water, they don'tconduct electricity.
Community Answer
Properties of covalent compound?
Properties of Covalent Compounds
Covalent compounds, also known as molecular compounds, are formed when two or more nonmetals share electrons in a covalent bond. These compounds have a unique set of properties that distinguish them from other types of compounds such as ionic compounds. Let's explore the properties of covalent compounds in detail.
1. **Low Melting and Boiling Points**: Covalent compounds generally have lower melting and boiling points compared to ionic compounds. This is because covalent bonds are relatively weaker than ionic bonds, which require more energy to break.
2. **Low Solubility in Water**: Many covalent compounds are insoluble or have low solubility in water. This is because water, being a polar molecule, can only dissolve other polar or ionic substances. Covalent compounds, consisting of nonpolar molecules, do not readily dissolve in water.
3. **Nonconductivity**: Covalent compounds are typically poor conductors of electricity in both solid and liquid states. This is because covalent compounds do not have free ions or charged particles that can carry an electric current.
4. **Low Hardness**: Covalent compounds are generally softer compared to ionic compounds. This is because the covalent bonds between atoms are relatively weaker and more flexible, allowing the compound to be easily scratched or deformed.
5. **Lower Density**: Covalent compounds tend to have lower densities compared to ionic compounds. This is because the atoms in covalent compounds are held together by shared electrons, resulting in a more dispersed structure.
6. **Odor and Color**: Covalent compounds often exhibit distinct odors and colors due to the presence of different atoms and functional groups. For example, various organic compounds have characteristic smells, and certain covalent compounds display vibrant colors in solution or solid form.
7. **Variable State of Matter**: Covalent compounds can exist as gases, liquids, or solids at room temperature, depending on the strength of the intermolecular forces. The weaker the intermolecular forces, the more likely the compound will be a gas or liquid.
8. **Low Electrical Conductivity**: Covalent compounds do not conduct electricity in their solid state, as there are no mobile charged particles. However, some covalent compounds, such as acids and certain organic compounds, can conduct electricity when dissolved in water or in molten form.
In summary, covalent compounds possess distinct properties such as low melting and boiling points, low solubility in water, nonconductivity, low hardness, lower density, odor and color, variable states of matter, and low electrical conductivity. These properties arise from the nature of covalent bonds and the arrangement of covalent compounds' molecules or atoms.
Covalent compounds, also known as molecular compounds, are formed when two or more nonmetals share electrons in a covalent bond. These compounds have a unique set of properties that distinguish them from other types of compounds such as ionic compounds. Let's explore the properties of covalent compounds in detail.
1. **Low Melting and Boiling Points**: Covalent compounds generally have lower melting and boiling points compared to ionic compounds. This is because covalent bonds are relatively weaker than ionic bonds, which require more energy to break.
2. **Low Solubility in Water**: Many covalent compounds are insoluble or have low solubility in water. This is because water, being a polar molecule, can only dissolve other polar or ionic substances. Covalent compounds, consisting of nonpolar molecules, do not readily dissolve in water.
3. **Nonconductivity**: Covalent compounds are typically poor conductors of electricity in both solid and liquid states. This is because covalent compounds do not have free ions or charged particles that can carry an electric current.
4. **Low Hardness**: Covalent compounds are generally softer compared to ionic compounds. This is because the covalent bonds between atoms are relatively weaker and more flexible, allowing the compound to be easily scratched or deformed.
5. **Lower Density**: Covalent compounds tend to have lower densities compared to ionic compounds. This is because the atoms in covalent compounds are held together by shared electrons, resulting in a more dispersed structure.
6. **Odor and Color**: Covalent compounds often exhibit distinct odors and colors due to the presence of different atoms and functional groups. For example, various organic compounds have characteristic smells, and certain covalent compounds display vibrant colors in solution or solid form.
7. **Variable State of Matter**: Covalent compounds can exist as gases, liquids, or solids at room temperature, depending on the strength of the intermolecular forces. The weaker the intermolecular forces, the more likely the compound will be a gas or liquid.
8. **Low Electrical Conductivity**: Covalent compounds do not conduct electricity in their solid state, as there are no mobile charged particles. However, some covalent compounds, such as acids and certain organic compounds, can conduct electricity when dissolved in water or in molten form.
In summary, covalent compounds possess distinct properties such as low melting and boiling points, low solubility in water, nonconductivity, low hardness, lower density, odor and color, variable states of matter, and low electrical conductivity. These properties arise from the nature of covalent bonds and the arrangement of covalent compounds' molecules or atoms.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
Properties of covalent compound?
Question Description
Properties of covalent compound? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Properties of covalent compound? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Properties of covalent compound?.
Properties of covalent compound? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Properties of covalent compound? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Properties of covalent compound?.
Solutions for Properties of covalent compound? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of Properties of covalent compound? defined & explained in the simplest way possible. Besides giving the explanation of
Properties of covalent compound?, a detailed solution for Properties of covalent compound? has been provided alongside types of Properties of covalent compound? theory, EduRev gives you an
ample number of questions to practice Properties of covalent compound? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























