JEE Exam > JEE Questions > Among the following, the correct statement(s)...
Start Learning for Free
Among the following, the correct statement(s) is are
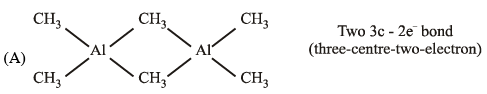
- a)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structure
- b)AlCl3 has the three-centre two-electron bonds in its dimeric structure
- c)BH3 has the three-centre two-electron bonds in its dimeric structure
- d)The Lewis acidity of BCl3 is greater than that of AlCl3
Correct answer is option 'A,C,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Among the following, the correct statement(s) is area)Al(CH3)3 has the...
Ans. (ACD)
(A)

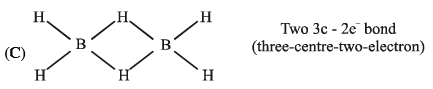
(D)
(A)
(D)
Lewis acidic strength decreases down the group. The decrease in acid strength occurs because as size increases, the attraction between the incoming electron pair and the nucleus weakens. Hence Lewis acidic strength of BCl3 is more than AlCl3.
Most Upvoted Answer
Among the following, the correct statement(s) is area)Al(CH3)3 has the...
Explanation:
a) Al(CH3)3 has the three-centre two-electron bonds in its dimeric structure:
- Al(CH3)3 is a Lewis acid and can form dimeric structures through the coordination of the Al center with the lone pair of electrons on the methyl groups.
- In the dimeric structure, three Al(CH3)3 units come together to form a cyclic trimer with Al atoms at the vertices of an equilateral triangle.
- Each Al atom is bonded to three methyl groups and shares its three valence electrons with the other two Al atoms, forming three-center two-electron bonds.
- These three-center two-electron bonds are formed by the overlap of the filled p-orbitals on the methyl carbon atoms and the empty p-orbitals on the Al atoms.
b) AlCl3 does not have three-centre two-electron bonds in its dimeric structure:
- AlCl3 is a Lewis acid and can form dimeric structures, but it does not have three-center two-electron bonds in its dimeric structure.
- In the dimeric structure, two AlCl3 units come together through the coordination of the Cl atoms with the Al center of the other unit.
- Each Al atom is bonded to six Cl atoms, forming octahedral coordination geometry.
- There are no three-center two-electron bonds present in the dimeric structure of AlCl3.
c) BH3 has the three-centre two-electron bonds in its dimeric structure:
- BH3 is a Lewis acid and can form dimeric structures through the coordination of the B center with the lone pair of electrons on the hydrogen atoms.
- In the dimeric structure, two BH3 units come together to form a cyclic dimer with B atoms at the vertices of a hexagon.
- Each B atom is bonded to two hydrogen atoms and shares its three valence electrons with the other B atom, forming three-center two-electron bonds.
- These three-center two-electron bonds are formed by the overlap of the filled p-orbitals on the hydrogen atoms and the empty p-orbitals on the B atoms.
d) The Lewis acidity of BCl3 is greater than that of AlCl3:
- The Lewis acidity of a compound is a measure of its ability to accept a lone pair of electrons and form a coordinate bond.
- BCl3 has a higher Lewis acidity than AlCl3 because boron is a smaller atom than aluminum, resulting in a higher charge density on the B atom.
- The higher charge density on the B atom allows it to more effectively attract and accept a lone pair of electrons, making BCl3 a stronger Lewis acid compared to AlCl3.
a) Al(CH3)3 has the three-centre two-electron bonds in its dimeric structure:
- Al(CH3)3 is a Lewis acid and can form dimeric structures through the coordination of the Al center with the lone pair of electrons on the methyl groups.
- In the dimeric structure, three Al(CH3)3 units come together to form a cyclic trimer with Al atoms at the vertices of an equilateral triangle.
- Each Al atom is bonded to three methyl groups and shares its three valence electrons with the other two Al atoms, forming three-center two-electron bonds.
- These three-center two-electron bonds are formed by the overlap of the filled p-orbitals on the methyl carbon atoms and the empty p-orbitals on the Al atoms.
b) AlCl3 does not have three-centre two-electron bonds in its dimeric structure:
- AlCl3 is a Lewis acid and can form dimeric structures, but it does not have three-center two-electron bonds in its dimeric structure.
- In the dimeric structure, two AlCl3 units come together through the coordination of the Cl atoms with the Al center of the other unit.
- Each Al atom is bonded to six Cl atoms, forming octahedral coordination geometry.
- There are no three-center two-electron bonds present in the dimeric structure of AlCl3.
c) BH3 has the three-centre two-electron bonds in its dimeric structure:
- BH3 is a Lewis acid and can form dimeric structures through the coordination of the B center with the lone pair of electrons on the hydrogen atoms.
- In the dimeric structure, two BH3 units come together to form a cyclic dimer with B atoms at the vertices of a hexagon.
- Each B atom is bonded to two hydrogen atoms and shares its three valence electrons with the other B atom, forming three-center two-electron bonds.
- These three-center two-electron bonds are formed by the overlap of the filled p-orbitals on the hydrogen atoms and the empty p-orbitals on the B atoms.
d) The Lewis acidity of BCl3 is greater than that of AlCl3:
- The Lewis acidity of a compound is a measure of its ability to accept a lone pair of electrons and form a coordinate bond.
- BCl3 has a higher Lewis acidity than AlCl3 because boron is a smaller atom than aluminum, resulting in a higher charge density on the B atom.
- The higher charge density on the B atom allows it to more effectively attract and accept a lone pair of electrons, making BCl3 a stronger Lewis acid compared to AlCl3.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer?
Question Description
Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer?.
Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer?.
Solutions for Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer?, a detailed solution for Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? has been provided alongside types of Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Among the following, the correct statement(s) is area)Al(CH3)3 has the three-centre two-electron bonds in its dimeric structureb)AlCl3 has the three-centre two-electron bonds in its dimeric structurec)BH3 has the three-centre two-electron bonds in its dimeric structured)The Lewis acidity of BCl3 is greater than that of AlCl3Correct answer is option 'A,C,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























