Chemistry Exam > Chemistry Questions > 0.1 M NaCl and 0.05 M BaCl2 solutions are sep...
Start Learning for Free
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:
- a)There is no movement of any solution across the membrane
- b)Water flows from BaCl2 solution towards NaCl solution
- c)Water flows from NaCl solution towards BaCl2 solution
- d)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeabl...
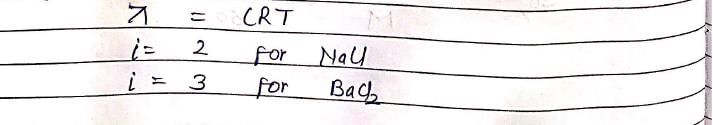

Most Upvoted Answer
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeabl...
Explanation:
Osmosis is the movement of solvent molecules across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. In this case, water molecules will move from the region of lower solute concentration (0.1 M NaCl) to the region of higher solute concentration (0.05 M BaCl2).
The correct answer is option 'B', which states that water flows from BaCl2 solution towards NaCl solution.
Reasoning:
To understand why water moves from BaCl2 solution towards NaCl solution, we need to calculate the osmotic pressure of each solution.
Osmotic pressure (π) is defined as the pressure required to prevent the movement of solvent molecules across a semi-permeable membrane. It is given by the equation:
π = MRT
where M is the molar concentration of the solute, R is the gas constant, and T is the temperature in Kelvin.
Let's calculate the osmotic pressure of each solution:
Osmotic pressure of NaCl solution:
M = 0.1 M
R = 0.082 L atm/mol K
T = 298 K
π = MRT
π = (0.1)(0.082)(298)
π = 2.44 atm
Osmotic pressure of BaCl2 solution:
M = 0.05 M (Note that BaCl2 dissociates into three ions in solution: Ba2+, 2Cl-. Hence, the effective molarity of BaCl2 is 0.15 M)
R = 0.082 L atm/mol K
T = 298 K
π = MRT
π = (0.15)(0.082)(298)
π = 3.66 atm
Since the osmotic pressure of BaCl2 solution is higher than that of NaCl solution, water molecules will move from BaCl2 solution towards NaCl solution to equalize the concentration of solutes on both sides of the membrane.
Therefore, option 'B' is the correct answer.
Osmosis is the movement of solvent molecules across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. In this case, water molecules will move from the region of lower solute concentration (0.1 M NaCl) to the region of higher solute concentration (0.05 M BaCl2).
The correct answer is option 'B', which states that water flows from BaCl2 solution towards NaCl solution.
Reasoning:
To understand why water moves from BaCl2 solution towards NaCl solution, we need to calculate the osmotic pressure of each solution.
Osmotic pressure (π) is defined as the pressure required to prevent the movement of solvent molecules across a semi-permeable membrane. It is given by the equation:
π = MRT
where M is the molar concentration of the solute, R is the gas constant, and T is the temperature in Kelvin.
Let's calculate the osmotic pressure of each solution:
Osmotic pressure of NaCl solution:
M = 0.1 M
R = 0.082 L atm/mol K
T = 298 K
π = MRT
π = (0.1)(0.082)(298)
π = 2.44 atm
Osmotic pressure of BaCl2 solution:
M = 0.05 M (Note that BaCl2 dissociates into three ions in solution: Ba2+, 2Cl-. Hence, the effective molarity of BaCl2 is 0.15 M)
R = 0.082 L atm/mol K
T = 298 K
π = MRT
π = (0.15)(0.082)(298)
π = 3.66 atm
Since the osmotic pressure of BaCl2 solution is higher than that of NaCl solution, water molecules will move from BaCl2 solution towards NaCl solution to equalize the concentration of solutes on both sides of the membrane.
Therefore, option 'B' is the correct answer.
Free Test
FREE
| Start Free Test |
Community Answer
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeabl...
The question is about Osmosis. In Osmosis water flows from *water's* high concentration to *water's* low concentration. That means from the lower concentration of solution to the higher concentration of solution. So the answer is Option B.
But before checking the concentration we need to see if the osmotic pressure is different or same for the sides. Because if osmotic pressure is same, there would be no net flow of water (i.e. isotonic solutions). So to calculate osmotic pressure the formula is π=i*c*R*T (i=van't hoff factor, c is concentration)
If you calculate π i.e. osmotic pressure, it's different for both the solutions. So there would be the flow of water n Concentration decides the net flow as I explained in the above para.
I hope it helps! :)

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer?
Question Description
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer?.
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer?.
Solutions for 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer?, a detailed solution for 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice 0.1 M NaCl and 0.05 M BaCl2 solutions are separated by a semi-permeable membrane in a container. For this system, choose the correct alternative:a)There is no movement of any solution across the membraneb)Water flows from BaCl2 solution towards NaCl solutionc)Water flows from NaCl solution towards BaCl2 solutiond)Osmotic pressure of 0.1 M NaCl solution is lower than the osmotic pressure of BaCl2Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















