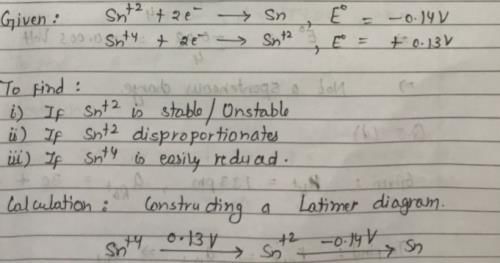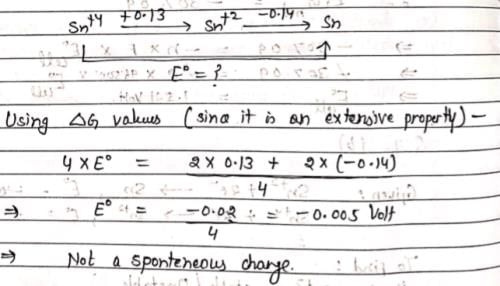Chemistry Exam > Chemistry Questions > Sn2+ + 2e– → Sn, E0 = – 0.1...
Start Learning for Free
Sn2+ + 2e– → Sn, E0 = – 0.14 V
Sn4+ + 2e- → Sn2+, E0 = + 0.13 V
Which of the following is true?
- a)Sn2+ is unstable and disproportionates to Sn4+ and Sn
- b)Sn2+ is stable and disproportionat ion reaction is not spontaneous
- c)Sn4+ is easily reduced to Sn
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+...
The term "SN2" refers to a type of nucleophilic substitution reaction that involves a simultaneous attack by a nucleophile and the departure of a leaving group.
The "S" stands for substitution, indicating that a new group is replacing the leaving group. The "N" stands for nucleophilic, indicating that the nucleophile (electron-rich species) is involved in the reaction. The "2" represents the bimolecular nature of the reaction, meaning that the rate-determining step involves two species colliding with each other.
In an SN2 reaction, the nucleophile approaches the carbon atom attached to the leaving group from the opposite side (backside attack) and displaces the leaving group. This concerted mechanism leads to inversion of stereochemistry at the carbon center.
The "2e" mentioned in your question could refer to the two electrons involved in the bond-making and bond-breaking process during the SN2 reaction. In this reaction, a nucleophile donates a pair of electrons to form a new bond while the leaving group accepts a pair of electrons to break a bond.
Overall, the SN2 reaction is characterized by a single-step mechanism, backside attack, inversion of stereochemistry, and involvement of a nucleophile and a leaving group.
The "S" stands for substitution, indicating that a new group is replacing the leaving group. The "N" stands for nucleophilic, indicating that the nucleophile (electron-rich species) is involved in the reaction. The "2" represents the bimolecular nature of the reaction, meaning that the rate-determining step involves two species colliding with each other.
In an SN2 reaction, the nucleophile approaches the carbon atom attached to the leaving group from the opposite side (backside attack) and displaces the leaving group. This concerted mechanism leads to inversion of stereochemistry at the carbon center.
The "2e" mentioned in your question could refer to the two electrons involved in the bond-making and bond-breaking process during the SN2 reaction. In this reaction, a nucleophile donates a pair of electrons to form a new bond while the leaving group accepts a pair of electrons to break a bond.
Overall, the SN2 reaction is characterized by a single-step mechanism, backside attack, inversion of stereochemistry, and involvement of a nucleophile and a leaving group.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer?
Question Description
Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer?.
Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Sn2+ + 2e– → Sn, E0 = – 0.14 VSn4+ + 2e- → Sn2+, E0 = + 0.13 VWhich of the following is true?a)Sn2+ is unstable and disproportionates to Sn4+ and Snb)Sn2+ is stable and disproportionat ion reaction is not spontaneousc)Sn4+ is easily reduced to Snd)None of theseCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.