6 Days Study Plan: Structure of Atom | Chemistry Class 11 - NEET PDF Download
Let's explore the Class 11 Chemistry chapter called "Structure of Atom" and understand how it's important for the NEET exam. By studying the past years NEET questions (from 2016 to 2025), we can see that this chapter is crucial in the exam. If you want to score well in NEET, you need to understand the concepts in this chapter.
Topics Covered in the Chapter
- Electromagnetic Radiation: Wave & Particle Nature
- Atomic Models: Thomson & Rutherford
- Atomic Number & Mass Number, Isotopes & Isobars
- Atomic Spectrum & Bohr's Model of Hydrogen Atom
- Electronic Configuration & Wave functions
- Dual Nature of Matter & Heisenberg's Uncertainty Principle*
- Quantum Mechanical Model of Atom
- Quantum Numbers & Concept of Orbitals
- Aufbau's Principle, Pauli's Principle & Hund's Principle
Now, let's create a 5-day study plan to master these topics and an additional day for revision.
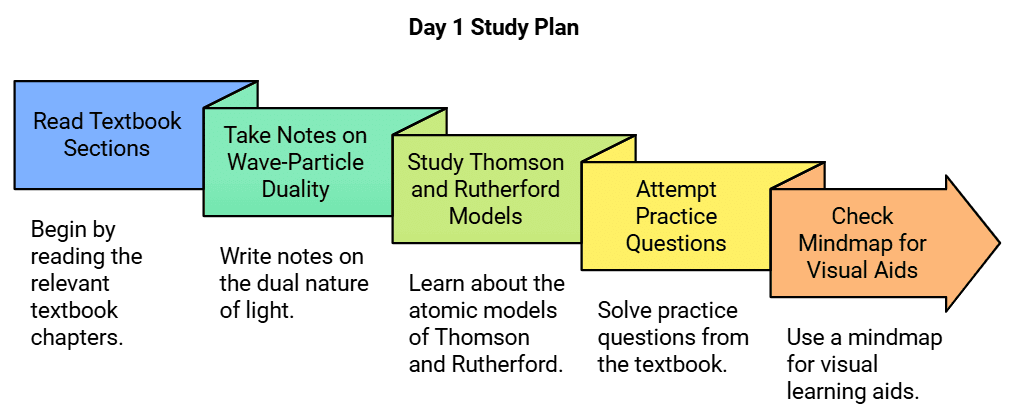
Day 1: Electromagnetic Radiation, Atomic Models: Thomson & Rutherford
- Start your day by reading the corresponding sections from the NCERT Chemistry Class 11 textbook.
- Take notes on the wave-particle duality of electromagnetic radiation.
- Study the atomic models proposed by Thomson and Rutherford.
- Attempt practice questions from the NCERT textbook.
- Check out the Mindmap for visual aids.
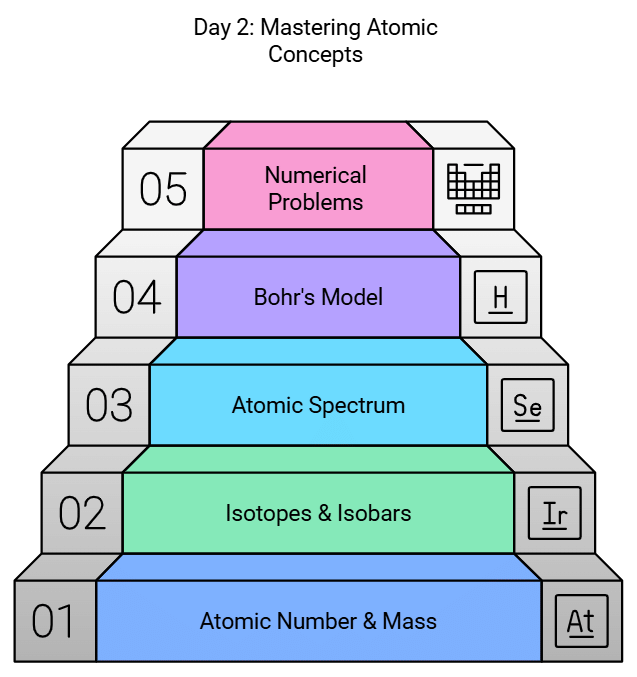
Day 2: Atomic Number & Mass Number, Isotopes & Isobars, Atomic Spectrum & Bohr's Model of Hydrogen Atom
- Focus on the concepts of atomic number, mass number, isotopes, and isobars.
- Study the atomic spectrum and Bohr's model of the hydrogen atom.
- Solve numerical problems related to these topics.
- Access important notes of the chapter for quick revision.
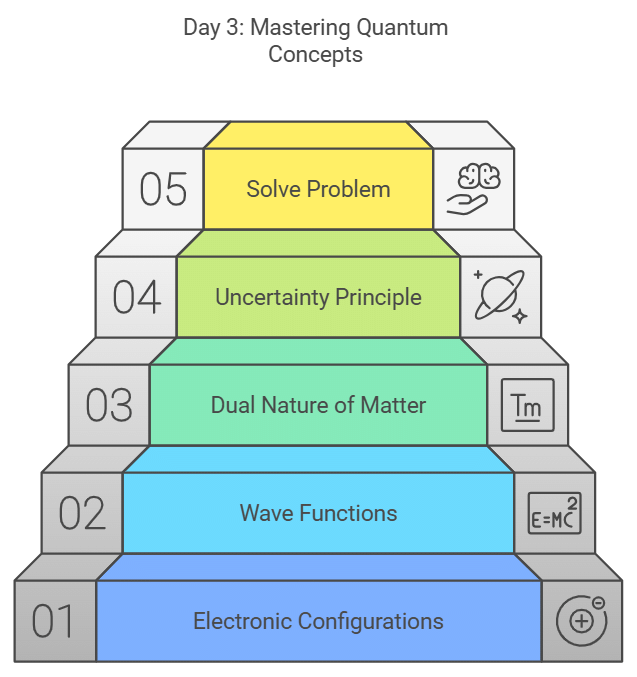
Day 3: Electronic Configuration & Wave functions, Dual Nature of Matter & Heisenberg's Uncertainty Principle
- Dive into electronic configuration and wave functions.
- Understand the dual nature of matter and Heisenberg's uncertainty principle.
- Practice solving problems related to electronic configurations.
- Take the PPT for additional insights.
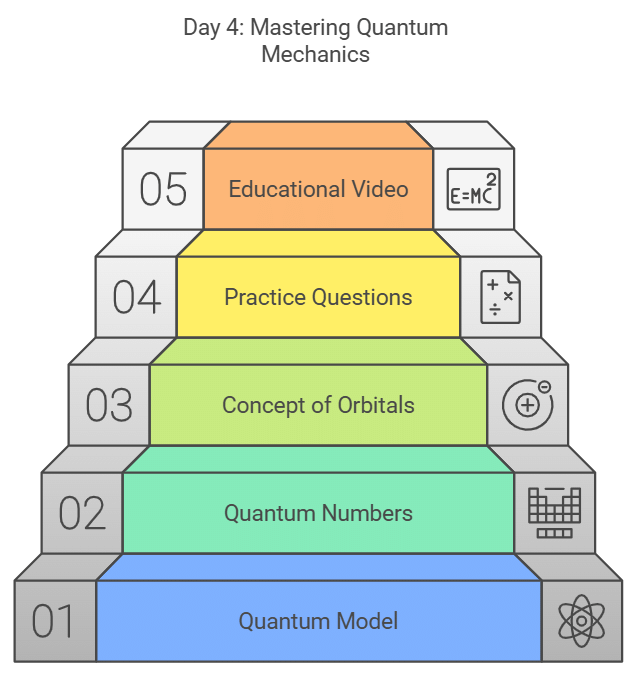
Day 4: Quantum Mechanical Model of Atom, Quantum Numbers & Concept of Orbitals
- Study the quantum mechanical model of the atom.
- Learn about quantum numbers and the concept of orbitals.
- Attempt practice questions from the NCERT textbook.
- Check out the video on the history of atoms for a visual explanation.
Day 5: Aufbau's Principle, Pauli's Principle & Hund's Principle
- Finish the chapter by focusing on Aufbau's principle, Pauli's principle, and Hund's principle.
- Review all key concepts and important formulas from the chapter.
- Take a few practice quizzes on EduRev to reinforce your knowledge.
- Prepare for the revision day.
Day 6: Revision
- Allocate this day solely for revision.
- Go through your notes and important formulas.
- Solve sample questions from the NCERT textbook and EduRev.
- Refresh your understanding of the entire chapter.
- Review how the chapter is important for NEET by checking the NEET previous year questions.
By following this study plan, you'll cover all the topics comprehensively and get ample practice. Remember to refer to the NCERT Chemistry Class 11 textbook and use the resources available on EduRev to enhance your understanding of the chapter. Good luck with your studies!
Here are all the important links and topic links for the chapter "Structure of Atom" summarized at the end:
Important Links:
- NEET Exam - Explore the NEET exam details.
- NEET Previous Year Questions (2016-2025) - Practice previous years' NEET questions.
- Mindmap - Visualize key concepts using a mind map.
- Important Notes of the chapter - Access important notes for quick revision.
- PPT - Review the chapter through a presentation.
- NCERT Textbook - Refer to the NCERT Chemistry Class 11 textbook.
- NCERT Solutions - Find solutions to NCERT textbook questions.
- Video: History of Atoms - Watch a video on the history of atoms.
- Test: Electromagnetic Radiations - Practice a test on electromagnetic radiations.
- Chapter: Structure of Atom - Access the complete chapter on EduRev.
You can use these links to access specific resources and topics related to the chapter. Good luck with your studies!
|
114 videos|263 docs|74 tests
|
FAQs on 6 Days Study Plan: Structure of Atom - Chemistry Class 11 - NEET
| 1. What is electromagnetic radiation and how does it relate to atomic models? |  |
| 2. What are isotopes and how do they differ from isobars? |  |
| 3. What is the significance of Bohr's model of the hydrogen atom? |  |
| 4. How do quantum numbers define the properties of electrons in an atom? |  |
| 5. What are Aufbau's Principle, Pauli's Principle, and Hund's Principle in electronic configuration? |  |






















