Acids, Bases and Salts - 1 Class 10 Worksheet Science Chapter 2
Q1: Identify the basic salt from the following salts (1 Marks):
(a) Na2CO3
(b) NH4Cl
(c) NaNO3
(d) KCl
Ans: (a)
Na2CO3 is a basic salt
- Basic salts are formed when a strong base reacts with a weak acid. Sodium carbonate is the product of the reaction of a strong base (sodium hydroxide) with a weak acid (carbonic acid), and it has basic properties in solution.
- NH4Cl (Ammonium chloride) is an acidic salt.
- NaNO3 (Sodium nitrate) is a neutral salt.
- KCl (Potassium chloride) is also a neutral salt.
Q2: Read the passage and answer the following questions.
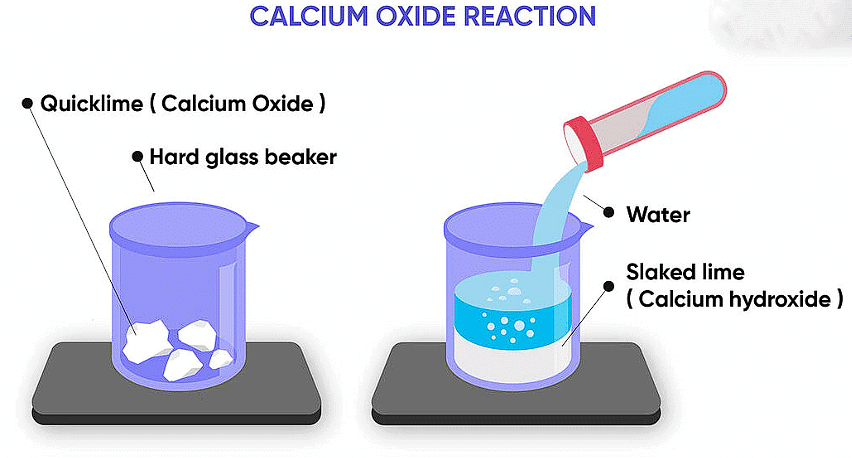
Suhana wanted her house to be whitewashed. She bought 10 kg of quicklime from the market and dissolved it in 30 L of water. On adding lime to water, she observed that the water started boiling even when it was not heated.
(a) _________ is formed when water is added to quicklime. (1 Marks)
(b) Write the reaction involved. (1 Marks)
(c) The product formed is acidic or basic in nature? (1 Marks)
(d) Which of the following statements is correct about the above reaction based on your observations? (1 Marks)
I. It is an endothermic reaction.
II. It is an exothermic reaction
III. The pH of the resulting solution will be more than seven.
IV. The pH of the resulting solution will be less than seven.
(i) I and II
(ii) II and III
(iii) I and IV
(iv) III and IV
Ans:
(a) Calcium hydroxide, Ca(OH)2
(b) CaO + H2O → Ca(OH)2 + Heat
(c) Calcium hydroxide is basic in nature.
(d) (ii) It is an exothermic reaction because heat is given out. The resulting compound is Ca(OH)2 which, is basic in nature. So the pH of the resulting solution will be more than seven.
Q3: Name a salt that does not contain water of crystallization. (1 Marks)
Ans: Baking soda, also known as sodium bicarbonate (NaHCO3), does not contain water of crystallization. It is a simple salt that is typically used in baking and other applications.

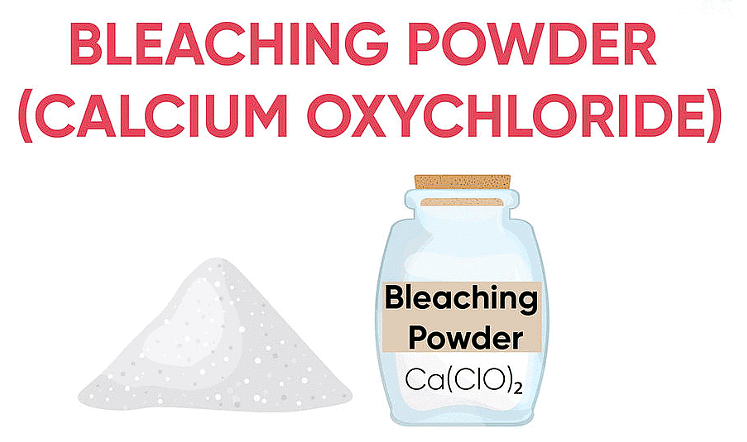
Q4: A sanitary worker uses a white chemical, having a strong smell of chlorine gas to disinfect the water tank. (3 Marks)
(i) Identify the chemical compound, write its chemical formula.
(ii) Give a chemical equation for its preparation.
(iii) Write its two uses other than disinfection.
Ans:
(i) Bleaching powder: CaOCl2
(ii) Ca(OH)2 + Cl2 → CaOCl2 + H2O
(iii) Two uses other than disinfection are:
- Paper industries
- Chemical Industries
Q5: Write the chemical name of Na2CO3.10H2O and Na2CO3. Write the significance of 10H2O. Mention the term used for water molecules attached with a salt. With the help of a chemical equation, explain the preparation of both Na2CO3.10H2O and Na2CO3. Also, list two uses of Na2CO3.10H2O. (5 Marks)
Ans: Na2CO3.10H2O: Sodium carbonate decahydrate.
Na2CO3: Anhydrous sodium carbonate.
10H2O: Water of crystallization which imparts shape and color to the crystals.
Preparation:
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Na2CO3 + 10H2O → Na2CO3.10H2O
Uses:
(i) Used in glass, soap and paper industries.
(ii) Used in the manufacture of borax.
(iii) Used as a cleansing agent for domestic purposes. (Any two)
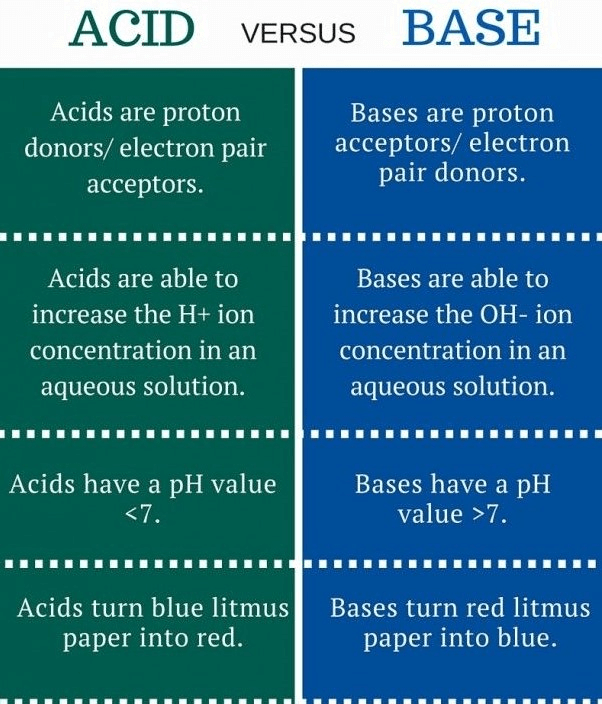
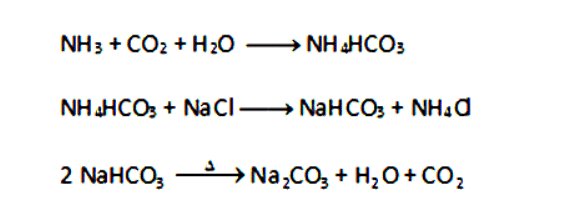 Q6: Write the main difference between an acid and a base. With the help of suitable examples, explain the term neutralization and the formation of:
Q6: Write the main difference between an acid and a base. With the help of suitable examples, explain the term neutralization and the formation of:
(i) acidic
(ii) basic and
(iii) neutral salts. (5 Marks)
Ans:
 (Any one)
(Any one)
Neutralization – A reaction of an acid with a base to produce salt and water.
(i) Acidic – NH4OH + HCl → NH4Cl + H2O
(ii) Basic – NaOH + H2CO3 → Na2CO3 + H2O
(iii) Neutral – KOH + HNO3 → KNO3 + H2O
(or any other example)
Q7: ‘Sweet tooth may lead to tooth decay.’ Explain why? What is the role of toothpaste in preventing cavities?
Ans: Sweet tooth leads to tooth decay, which is caused by bacteria's action on food particles remaining in the mouth, and acid is formed. As a result, the mouth's pH falls below 5.5, and the tooth enamel dissolves, resulting in cavities. Toothpaste is generally basic; they neutralize the excess acid produced in the mouth and prevent tooth decay.
Q8: Which has a higher concentration of H+ ions?
1 M HCl or 1 M CH3COOH. (1 Marks)
Ans: 1 M HCl has a higher concentration of H+ ions because when HCl dissolves in water, it dissociates completely into ions. At the same time, CH3COOH is a weak acid and does not dissociate into ions completely.
Q9: How is sodium hydroxide manufactured in industries? Name the process. In this process, a gas X is formed as a by-product. This gas reacts with lime water to give a compound Y, which is used as a bleaching agent in the chemical industry. Identify X and Y and write the chemical equation of the reactions involved. (3 Marks)
Ans: When electricity is passed through an aqueous sodium chloride solution (brine), it decomposes to form sodium hydroxide.
2NaCl (aq) (Sodium chloride) + 2H2O → 2NaOH (aq) (Sodium hydroxide)+ Cl2 (g) + H2(g)
This process is called the chlor-alkali process.
In the manufacture of sodium hydroxide, hydrogen gas and chlorine gas (X) are formed as byproducts. When chlorine gas (X) reacts with lime water, it forms calcium oxychloride (bleaching powder) Y.
The reaction is:
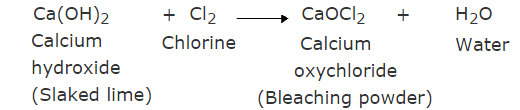
Y is Calcium oxychloride, which is used as a bleaching agent in the chemical industry.
Q11: Give suitable reasons for the following statements :
(i) Rainwater conducts electricity, but distilled water does not.
(ii) We feel a burning sensation in the stomach when we overeat.
(iii) A tarnished copper vessel regains its shine when rubbed with lemon.
(iv) The crystals of washing soda change to white powder on exposure to air.
(v) An aqueous sodium chloride solution is neutral, but an aqueous solution of sodium carbonate is basic. (5 Marks)
Ans:
(i) Distilled water does not conduct electricity because it does not contain any ionic compounds like acids, bases or salts dissolved.
(ii) When we overeat, an excess of acid is produced in the stomach, which causes a burning sensation.
(iii) Copper vessels tarnish due to the formation of basic copper carbonate, which gets neutralized when rubbed with lemon, and the copper vessel regains its shine.
(iv) Washing soda is sodium carbonate decahydrate which, when exposed to air, loses 10 molecules of water and changes to white powder.
(v) Sodium chloride is a salt of strong acid HCl and strong base NaOH, so it is neutral.
Sodium carbonate is a salt of weak acid H2CO3 and strong base NaOH, so it is basic.
Q12: What would be the color of litmus in a solution of sodium carbonate? (1 Marks)
Ans: The color of litmus in a solution of sodium carbonate is blue.
Q13: In one of the industrial processes used to manufacture sodium hydroxide, a gas X is formed as a by-product. The gas ‘X’ reacts with dry slaked lime to give a compound ‘Y,’ which is used as a bleaching agent in the textile industry. Identify X and Y. (3 Marks)
Ans:
(i) X = Chlorine gas, Y = Calcium oxychloride
(ii)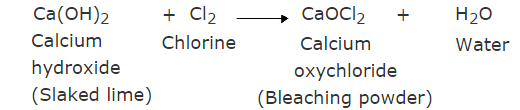
Q14: (a) Write the chemical formula of hydrated copper sulphate and anhydrous copper sulphate. Give an activity to illustrate how these two are interconvertible.
(b) Write chemical names and formulae of Plaster of Paris and gypsum. (5 Marks)
Ans:
(a) Hydrated copper sulphate – CuSO4.5H2O
Anhydrous copper sulphate – CuSO4.
Activity:
(i) Heat a few crystals of copper sulphate in a dry boiling tube.
(ii) Add 2 - 3 drops of water to the sample of copper sulphate obtained after heating.
After heating, water is removed, and salt turns white.
If crystals are moistened again with water, the blue colour reappears. The crystallization water is a fixed number of water molecules present in one formula unit of salt. Five water molecules are present in one formula unit of copper sulphate.
(b) Calcium sulphate hemihydrate – CaSO4.½H2O
Calcium sulphate dihydrate – CaSO4.2H2O.
Q15: (a) For the preparation of cakes, baking powder is used. If your mother uses baking soda instead of baking powder at home, how will it affect the cake's taste and why?
(b) How is baking soda converted into baking powder?
(c) What makes the cake soft and spongy? (1 Marks)
Ans:
(a) The cake will have a bitter taste because of Na2CO3 / sodium carbonate formation while baking/heating.
(b) By adding tartaric acid.
(c) The liberated CO2 gas.
|
80 videos|569 docs|80 tests
|
FAQs on Acids, Bases and Salts - 1 Class 10 Worksheet Science Chapter 2
| 1. What are some common examples of acids, bases, and salts? |  |
| 2. How do acids and bases differ in terms of their pH levels? |  |
| 3. What role do acids, bases, and salts play in everyday life? |  |
| 4. How can you differentiate between an acid, a base, and a salt based on their chemical properties? |  |
| 5. Can acids, bases, and salts be found naturally in the environment? |  |

















