Alcohols and Phenols: Chemical Reactions | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Chemical Reactions of Alcohols |

|
| Chemical Reactions of Phenol |

|
| Some Commercially Important Alcohols |

|
| 1. Methanol |

|
| 2. Ethanol |

|
Chemical reactions involving alcohols and phenols are essential aspects of organic chemistry, impacting numerous industrial processes and product synthesis. Alcohols, characterized by their hydroxyl (-OH) functional group attached to a carbon atom, and phenols, featuring a hydroxyl group directly bonded to an aromatic ring, undergo various transformations to yield a wide range of valuable compounds.
This document explains the key chemical reactions of alcohols and phenols, from basic transformations like oxidation and reduction to more specialized reactions such as esterification and ether formation.
Chemical Reactions of Alcohols
1. Reaction of Alcohols with Hydrogen Halides
When alcohols react with hydrogen halides (such as hydrogen chloride, HCl, or hydrogen bromide, HBr), they undergo a substitution reaction known as an alcohol halogenation reaction. The reaction involves the replacement of the hydroxyl (-OH) group of the alcohol with a halogen atom (-X).
General Reaction:
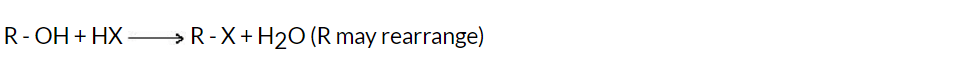
Reactivity of HX: Hl > HBr > HCl
Reactivity of ROH: allyl > benzyl > 3o > 2o> 1o
Mechanism: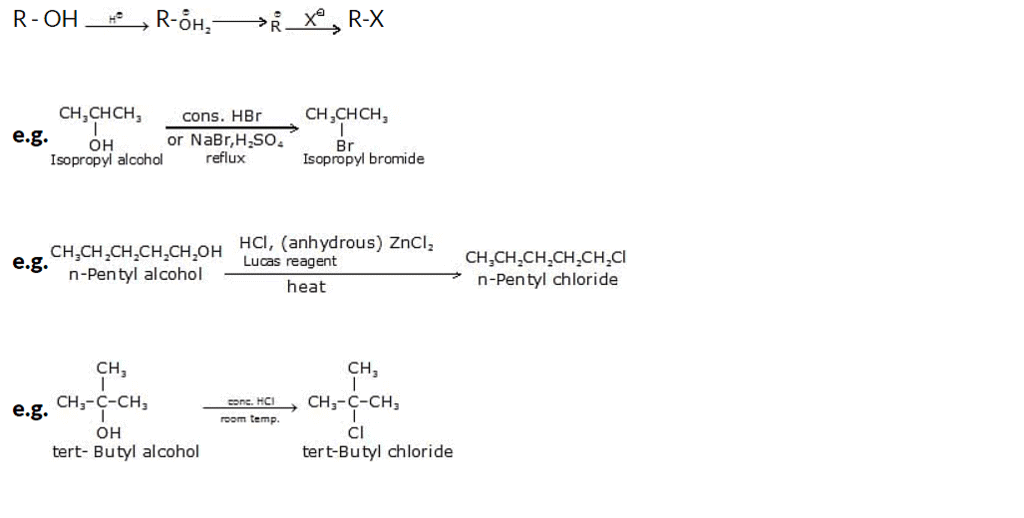
2. Reaction of Alcohols with Phosphorus Trihalides
When alcohols react with phosphorus trihalides (such as phosphorus trichloride, PCl3, or phosphorus tribromide, PBr3), they undergo a substitution reaction known as an alcohol phosphorane reaction. The reaction involves the replacement of the hydroxyl (-OH) group of the alcohol with a halogen atom (-X) and the formation of a phosphorus-containing group.
Phosphorus halides produce good yields of most primary and secondary alkyl halides, but none works well with tertiary alcohols. The two phosphorus halides used most often are PBr3 and the P4 / I2 combination.
General reaction:

Mechanism:
The mechanism for the reaction involves the attachment of the alcohol group on the phosphorus atom, displacing a bromide ion and forming a protonated alkyl dibromophosphite (see the following reaction).

In the second step, a bromide ion acts as a nucleophile to displace HOPBr2, a good leaving group due to the electronegative atoms bonded to the phosphorus.
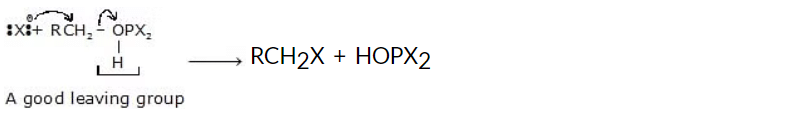
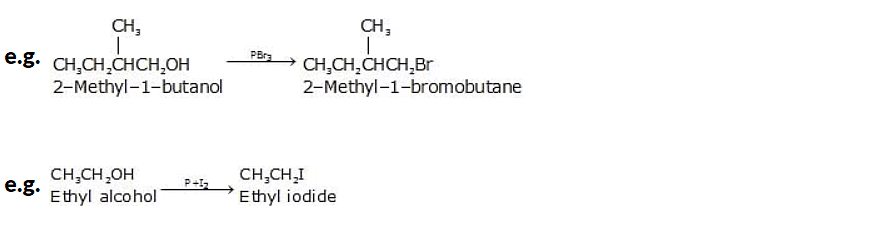
3. Reaction of Alcohols with Thionyl Chloride
When alcohols react with thionyl chloride (SOCl2), they undergo a substitution reaction known as an alcohol chlorination reaction. The reaction involves the replacement of the hydroxyl (-OH) group of the alcohol with a chlorine atom (-Cl) and the formation of sulfur dioxide gas (SO2) as a byproduct.
The general equation for the reaction of alcohols with thionyl chloride is:
ROH + SOCl2 → RCl + SO2 + HCl
where ROH represents the alcohol, SOCl2 represents thionyl chloride, RCl represents the alkyl chloride product, SO2 represents sulfur dioxide, and HCl represents hydrogen chloride.
4. Dehydration of Alcohols
Dehydration of alcohols is a chemical reaction in which water (H2O) is removed from the alcohol molecule, resulting in the formation of an alkene or an alkyne. This process involves the loss of a hydroxyl (-OH) group from the alcohol and a hydrogen atom from an adjacent carbon atom.
The general equation for the dehydration of an alcohol is
ROH → R-alkene + H2O
Mechanism
Step 1 :
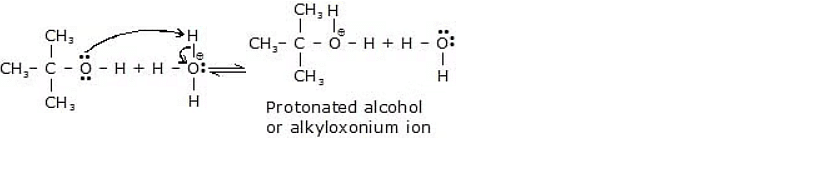 Step 2 :
Step 2 :

Step 3 :
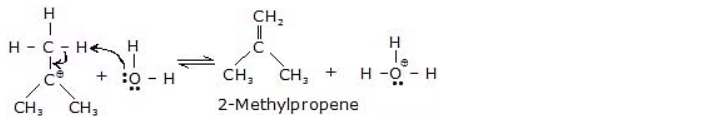
The reaction mechanism for the dehydration of alcohols can vary depending on the type of alcohol involved: primary (1°), secondary (2°), or tertiary (3°) alcohol.
Reactivity of ROH : 3º > 2º > 1º
5. Reaction of Alcohols with Metals
The reaction of alcohols with metals can vary depending on the specific metal and conditions involved. In general, the reaction between alcohols and metals can result in the formation of metal alkoxides and hydrogen gas. Here are a few examples:

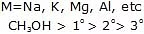
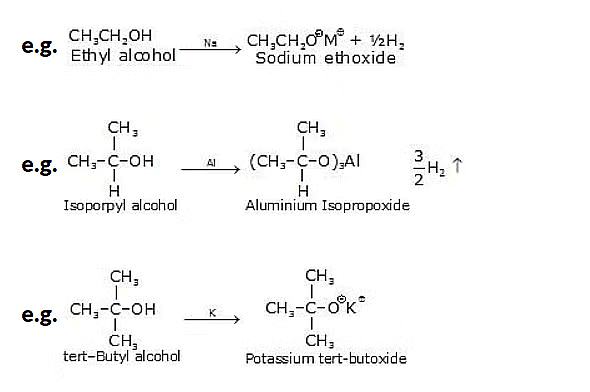
6. Ester Formation from Alcohols
Ester formation is a common reaction that involves the reaction between an alcohol and a carboxylic acid or its derivative. This reaction is known as esterification and results in the formation of an ester compound, along with the production of water as a byproduct.
General reaction:
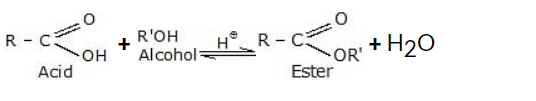
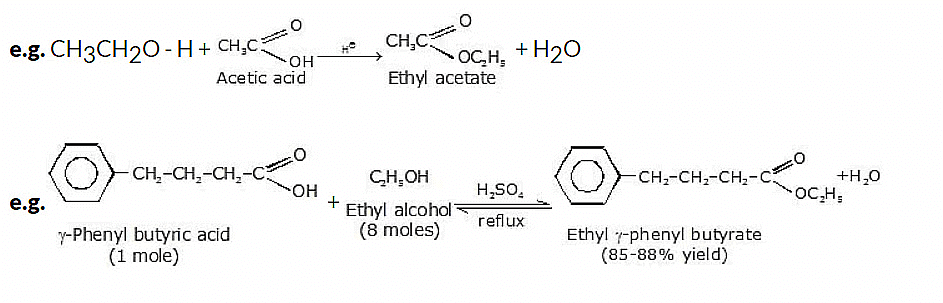
7. Oxidation Reactions of Alcohols
Oxidation reactions of alcohols involve the conversion of alcohol functional groups (-OH) into carbonyl groups (C=O). The degree of oxidation depends on the type of alcohol and the oxidizing agent used. There are three main types of alcohols: primary (1°), secondary (2°), and tertiary (3°), and each reacts differently with oxidizing agents.
(a) Oxidation of primary alcohols
Primary alcohols can undergo two levels of oxidation, depending on the reaction conditions and the oxidizing agent used.
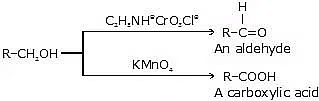
Aldehyde formation: Under controlled oxidation conditions, primary alcohols can be oxidized to aldehydes. Common oxidizing agents for this level of oxidation include pyridinium chlorochromate (PCC), Collins reagent, or selective oxidation methods. The reaction is often carried out under mild conditions to avoid further oxidation to carboxylic acids.
Alcohol to aldehyde: R-CH2OH → R-CHO + H2OCarboxylic acid formation: With stronger oxidizing agents, such as potassium permanganate (KMnO4) or chromium trioxide (CrO3) in acidic conditions, primary alcohols can be further oxidized to carboxylic acids.
Alcohol to carboxylic acid: R-CH2OH → R-COOH + H2O
(b) Oxidation of secondary alcohols
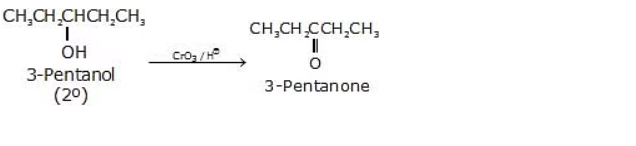
Secondary alcohols can be oxidized to ketones by a variety of oxidizing agents, including chromic acid (H2CrO4), potassium dichromate (K2Cr2O7) in acidic conditions, or pyridinium dichromate (PDC). The reaction is typically performed under acidic conditions to facilitate the formation of the carbonyl group without further oxidation to carboxylic acids.
The general equation for the oxidation of secondary alcohols is:
R2-CHOH → R2-CO + H2O
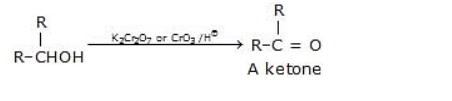
(c) Resistance of tertiary alcohols to oxidation
Tertiary alcohols do not undergo oxidation reactions under typical conditions because they lack a hydrogen atom on the carbon attached to the hydroxyl group. Since oxidation reactions involve the removal of a hydrogen atom from the alcohol, tertiary alcohols are not oxidized to aldehydes or carboxylic acids.

Below are some examples of Oxidation Reactions of primary alcohols
1. 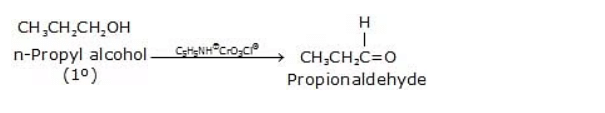 2.
2. 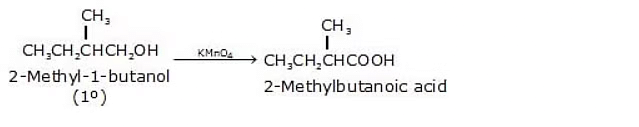 3.
3. 

Chemical Reactions of Phenol
1. Electrophilic Aromatic Substitution
Phenol is extremely reactive to electrophilic aromatic substitution as the oxygen atom's pi electrons give electron density into the ring. By this overall approach, several groups can be attached to the ring, through halogenation, sulfonation, acylation, and other methods. However, phenol's ring is so powerfully activated—second only to aniline—that chlorination or bromination of phenol will lead to replacement on all carbon atoms para and ortho to the hydroxy group, not only on one carbon.
Nitration: It reacts with dilute nitric acid at room temperature to produce a mixture of 2-nitrophenol and 4-nitrophenol. while with concentrated nitric acid, many nitro groups get replaced on the ring to produce 2, 4, 6-trinitrophenol which is also known as picric acid.
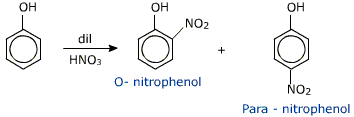 Phenol Reaction with Dilute Nitric Acid
Phenol Reaction with Dilute Nitric Acid
You can split the ortho and para isomers using steam distillation. o-Nitrophenol is steam-volatile because it forms hydrogen bonds within itself, making it easily evaporate. On the other hand, p-nitrophenol is less volatile because it engages in hydrogen bonding between different molecules, leading to their association.
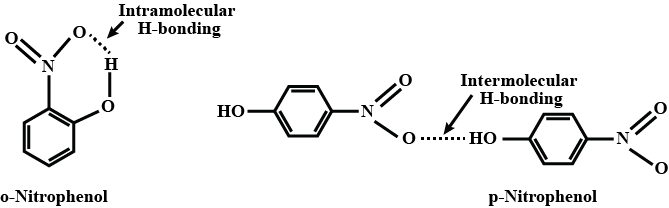 Separation of ortho and para nitrophenol
Separation of ortho and para nitrophenol
While with concentrated nitric acid, many nitro groups get replaced on the ring to produce 2, 4, 6-trinitrophenol which is also known as picric acid.
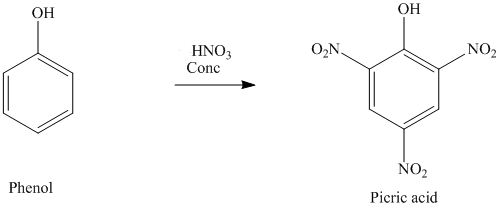 Phenol Reaction with Concentrated Nitric Acid
Phenol Reaction with Concentrated Nitric Acid
Halogenation: When phenol reacts with bromine, various reaction products emerge based on different experimental conditions.
a. When the reaction happens in less polar solvents like CHCl3 or CS2 at low temperatures, it leads to the formation of monobromophenols.
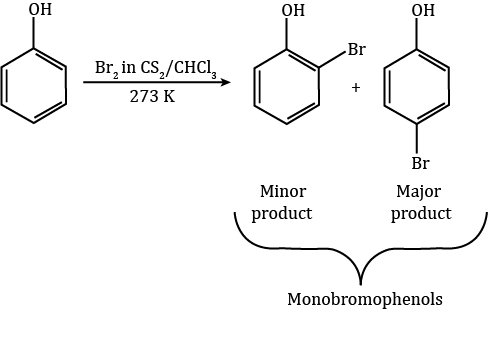 Phenol Reaction with Bromine in Less Polar Solvents
Phenol Reaction with Bromine in Less Polar Solvents
Typically, benzene halogenation requires a Lewis acid like FeBr3 to polarize the halogen molecule. However, with phenol, the bromine molecule gets polarized even without a Lewis acid. This happens because the –OH group attached to the benzene ring has a highly activating effect.
b. When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as a white precipitate.
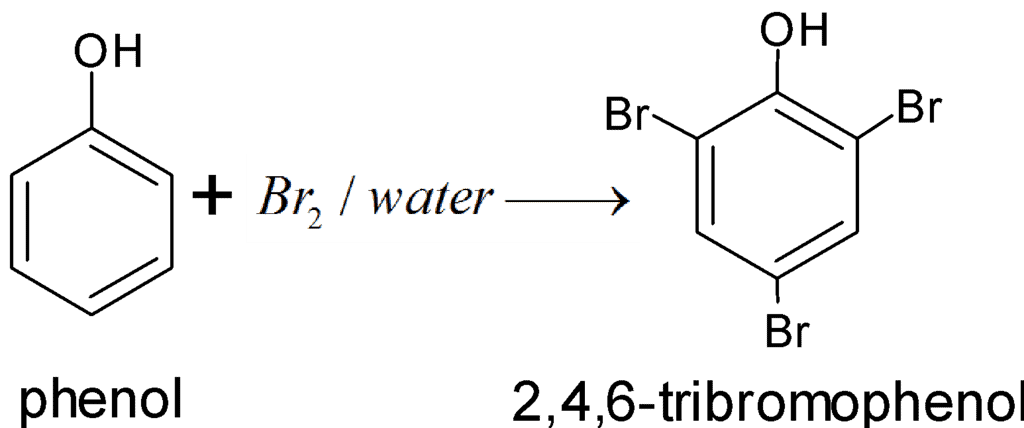 Phenol Reaction with Bromine Water
Phenol Reaction with Bromine Water
2. Kolbe Reaction
When phenol reacts with sodium hydroxide, it forms a phenoxide ion. Interestingly, this phenoxide ion is more reactive than phenol itself in electrophilic aromatic substitution reactions. Consequently, it readily undergoes substitution with carbon dioxide, which is a weak electrophile. The primary product of this reaction is orthohydroxybenzoic acid.
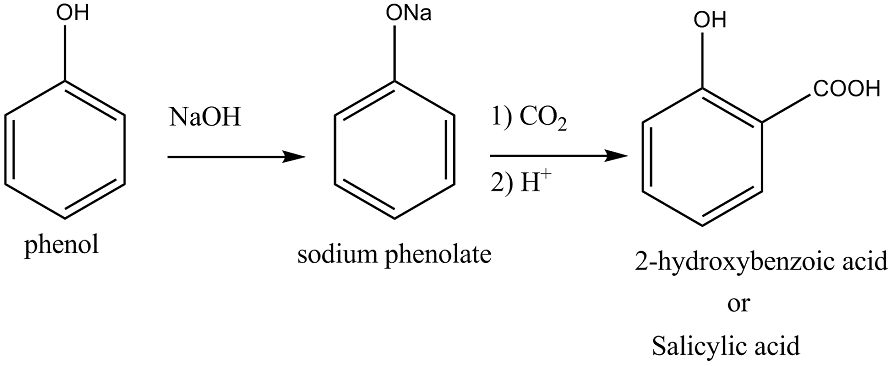 Kolbe's Reaction
Kolbe's Reaction
Mechanism of Kolbe's Reaction
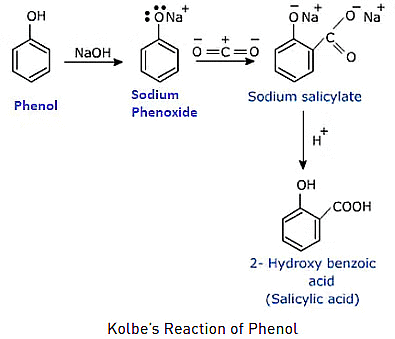 Mechanism of Kolbe's Reaction
Mechanism of Kolbe's Reaction
3. Reimer-Tiemann's Reaction
When phenol reacts with chloroform in the presence of sodium hydroxide, a –CHO group is added to the ortho position of the benzene ring. This chemical transformation is recognized as the Reimer-Tiemann reaction. The intermediate product, substituted benzal chloride, undergoes hydrolysis in the presence of alkali, resulting in the formation of salicylaldehyde.
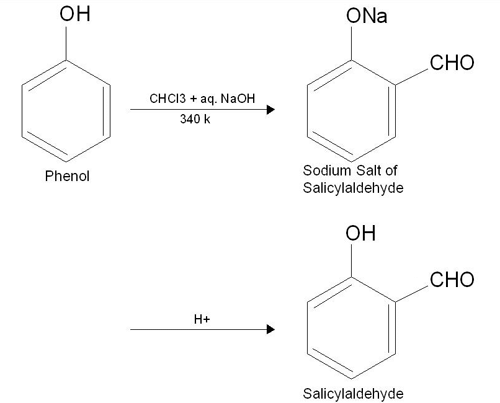 Reimer Tiemann Reaction
Reimer Tiemann Reaction
Mechanism of Reimer Tiemann's Reaction
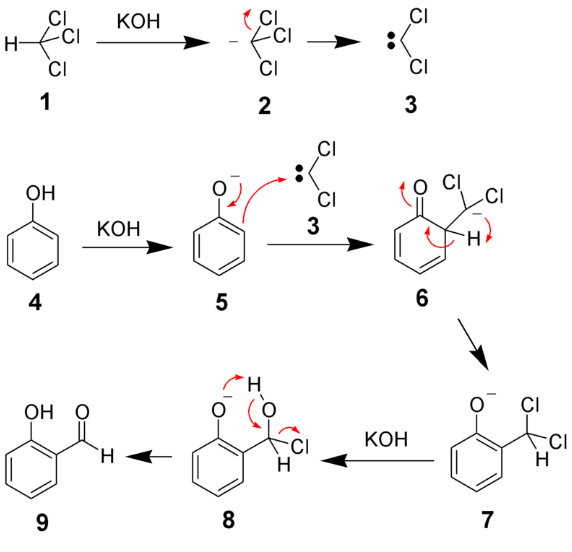 Reimer Tiemann Reaction Mechanism
Reimer Tiemann Reaction Mechanism
4. Reaction of Phenol with Zinc Dust
When phenol is heated with zinc dust, it undergoes a reaction that leads to the conversion of phenol into benzene.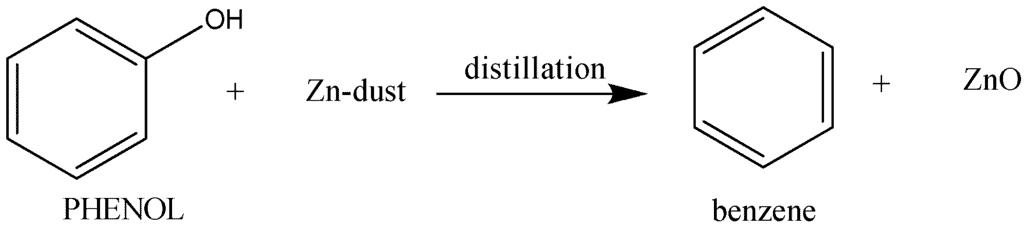 Reaction of Phenol with Zinc Dust
Reaction of Phenol with Zinc Dust
Mechanism of Phenol's Reaction with Zinc Dust
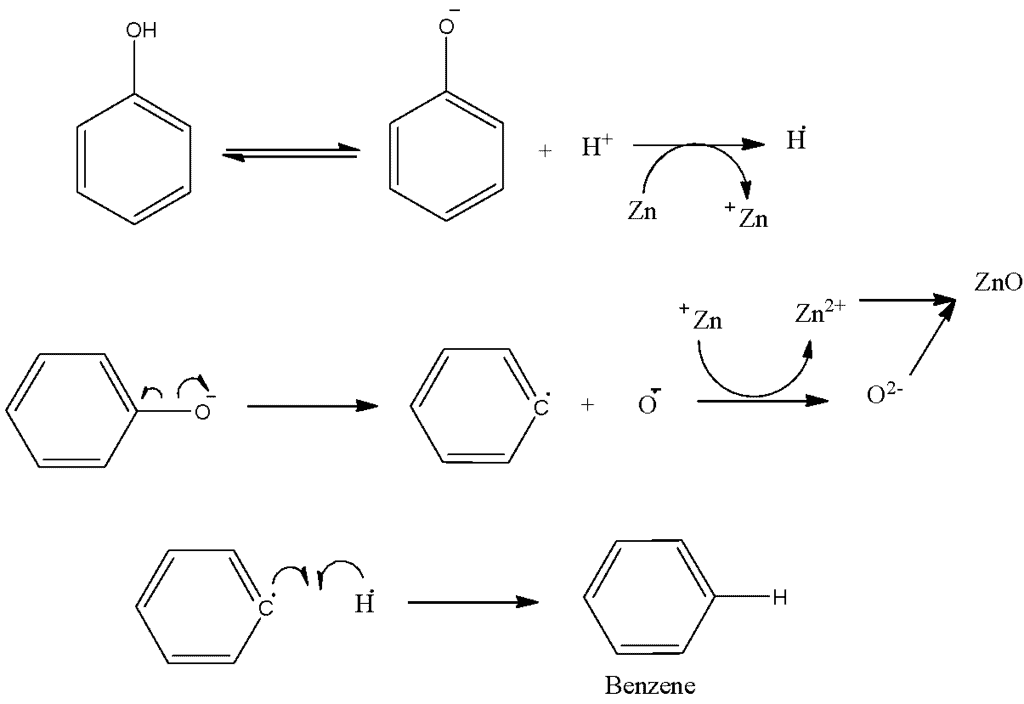 Mechanism of Phenol's Reaction with Zinc Dust
Mechanism of Phenol's Reaction with Zinc Dust
5. Oxidation
When phenol undergoes oxidation with chromic acid, it gives rise to a conjugated diketone called benzoquinone. In the presence of air, phenols experience a gradual oxidation, resulting in the formation of dark-colored mixtures that contain quinones.
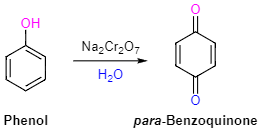 Oxidation of Phenol
Oxidation of Phenol
Some Commercially Important Alcohols
1. Methanol
Methanol, often referred to as 'wood spirit,' was initially obtained through the destructive distillation of wood. However, in contemporary times, the primary method for methanol production involves catalytic hydrogenation of carbon monoxide. This process occurs at elevated pressure and temperature, facilitated by a ZnO – Cr2O3 catalyst.
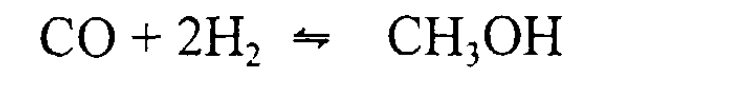 Methanol Production
Methanol Production
Methanol, a colorless liquid with a boiling point of 337 K, possesses a highly poisonous nature. Ingesting even small amounts of methanol can lead to blindness, while larger quantities can result in fatal consequences. Despite its hazardous properties, methanol finds utility as a solvent in paints and varnishes, and it is primarily employed in the production of formaldehyde.
2. Ethanol
Commercially, ethanol (C2H5OH) is obtained through fermentation, one of the oldest methods, particularly from sugars. In this process, sugar present in molasses, sugarcane, or fruits like grapes is converted into glucose and fructose, both having the formula C6H12O6. This conversion occurs in the presence of the enzyme invertase. The subsequent step involves the fermentation of glucose and fructose with the aid of another enzyme, zymase, found in yeast.
 Fermentation of Glucose
Fermentation of Glucose
In the art of winemaking, grapes play a dual role as the source of sugars and yeast. As grapes mature, the sugar content increases and yeast naturally grows on the outer skin. When grapes are crushed, sugar and enzymes come into contact, initiating fermentation. This process occurs in anaerobic conditions, without the presence of air, releasing carbon dioxide as a byproduct.
The activity of zymase, the enzyme involved in fermentation, ceases once the alcohol percentage exceeds 14 percent. Introducing air into the fermentation mixture can lead to the oxidation of ethanol to ethanoic acid, jeopardizing the flavor of alcoholic beverages.
Ethanol, a colorless liquid with a boiling point of 351 K, finds utility as a solvent in the paint industry and in the synthesis of various carbon compounds. To render commercial alcohol unfit for consumption, denaturation is employed, involving the addition of substances like copper sulfate for color and pyridine for an unpleasant odor.
In contemporary times, large-scale ethanol production predominantly involves the hydration of ethene.
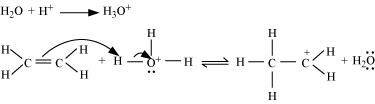 Hydration of Ethene
Hydration of Ethene
|
75 videos|278 docs|78 tests
|
FAQs on Alcohols and Phenols: Chemical Reactions - Chemistry Class 12 - NEET
| 1. What are some common chemical reactions of alcohols? |  |
| 2. How do phenols differ in their chemical reactions compared to alcohols? |  |
| 3. What are some commercially important alcohols and their uses? |  |
| 4. How do the chemical properties of alcohols and phenols affect their reactivity in different reactions? |  |
| 5. Can alcohols and phenols undergo similar chemical reactions? |  |















