Aldehydes, Ketones & Carboxylic Acids Class 12 Notes Chemistry Chapter 8
Aldehyde and Ketones
Preparation of Aldehydes
a. Oxidation of primary alcohols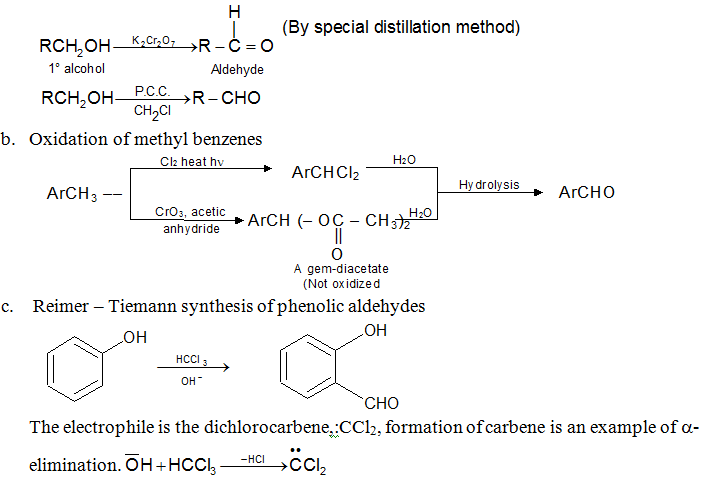
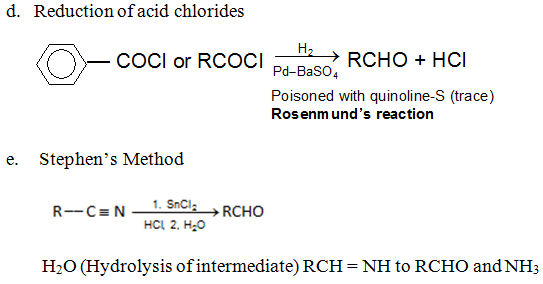
Preparation of Ketones:
a) Oxidation of Secondary alcohols: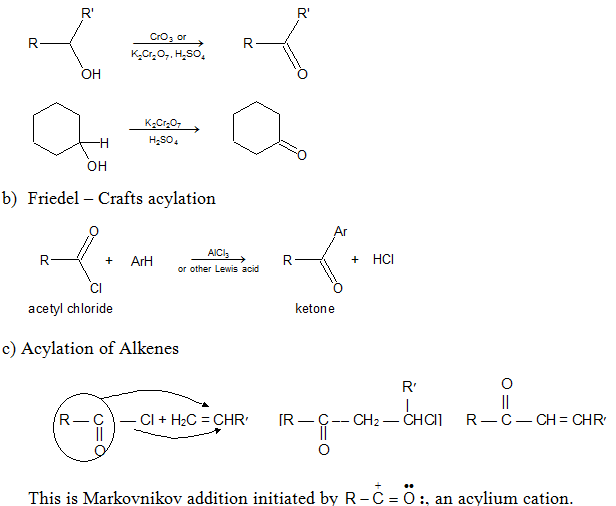
d) With Organometallics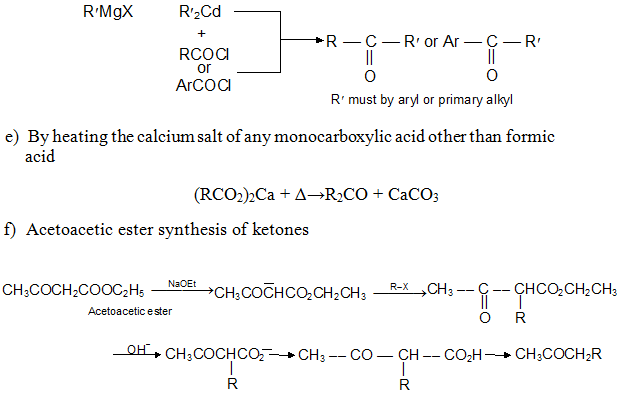
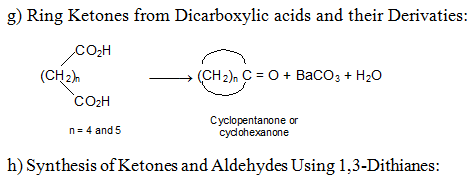
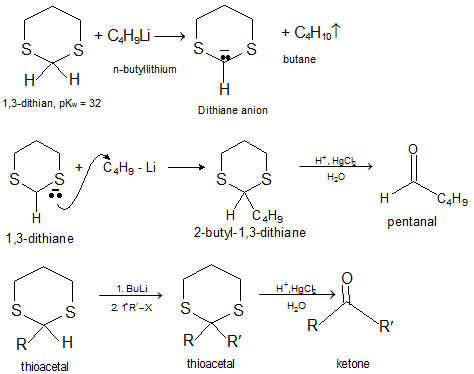
Reactions of Aldehydes and Ketones:
(a) Aldol condensation
Aldehydes and ketones having alpha hydrogen atom: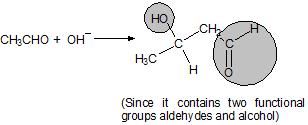
(b) Cannizzaro reaction:
Aldehydes and ketones having no alpha hydrogen atom: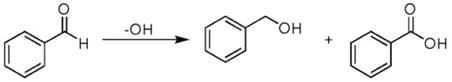
When two carbonyl groups are present within a molecule, think of intramolecular reaction.
OH- will attack more positively charged carbon. In this case, it is right >c=0 group.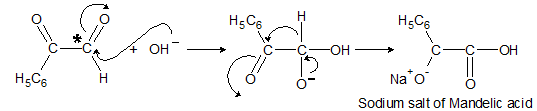
(c) Formation of Keto Esters
Esters having a-hydrogen on treatment with a strong base e.g. C2H5ONa. Undergo self condensation to produce b-keto esters. This reaction is Claisen Condensation.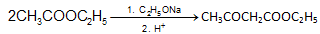
(d) Reformatsky Reaction
This is the reaction of a-haloester, usually an a-bromoester with an aldehyde or ketone in the presence of Zinc metal to produce b-hydroxyester.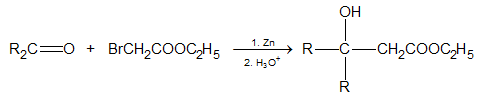
(e) Pinacol-pinacolone Rearrangement
The acid catalysed rearrangement of 1,2 diols (Vicinal diols) to aldehydes or ketones with the elimination of water is known as pinacol pinacolone rearrangement.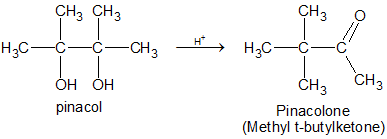
(a) Wittig-Ylide Reaction
Aldehydes and Ketones react with phosphorus Ylides to yield alkenes and triphenylphosphine oxide. An Ylide is a neutral molecule having a negative carbon adjacent to a positive hetero atom. Phosphorus ylides are also called phosphoranes.
(b) Preparation of Ylides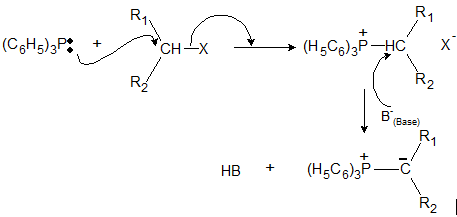
(c) Reaction of Ylide with >C=O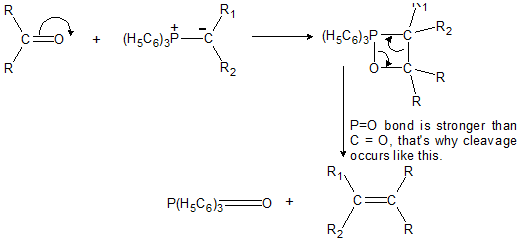
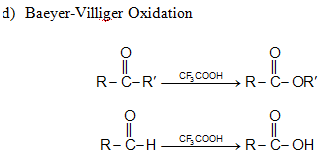
Above things happens in BVO (Bayer Villiger oxidation). Reagents are either per acetic acid or perbenzoic acid or pertrifluoroacetic acid or permonosulphuric acid.
(e) Addition of cyanide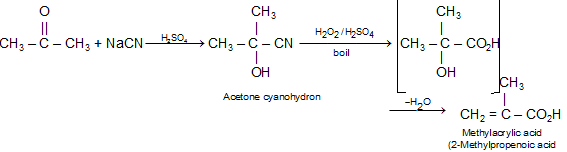
(f) Addition of bisulfite: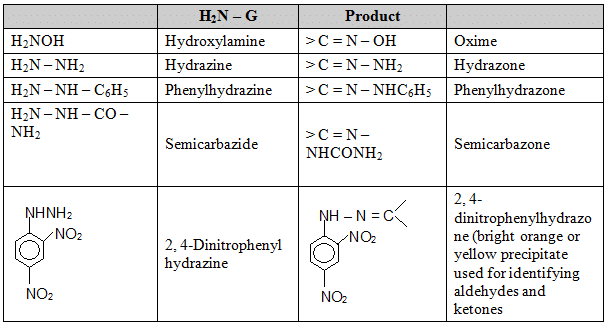
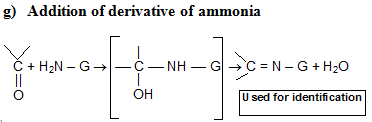
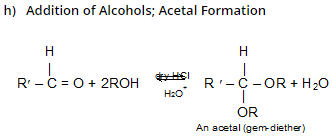
In H3O+, RCHO is regenerated because acetals undergo acid catalyzed cleavage much more easily than do ethers. Since acetals are stable in neutral or basic media, they are used to protect the – CH = O group.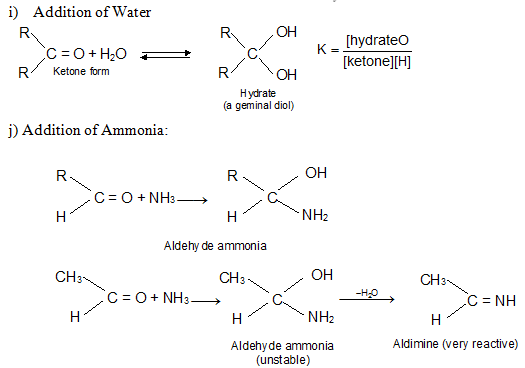
k) Tischenko reaction:
All aldehydes can be made to undergo the Cannizzaro reaction by treatment with aluminium ethoxide. Under these conditions the acids and alcohols are combined as the ester, and the reaction is then known as the Tischenko reaction; eg, acetaldehyde gives ethyl acetate, and propionaldehyde gives propyl propionate.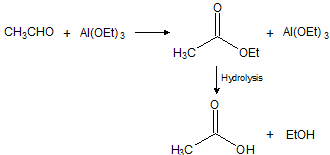
Oxidation of Aldehydes and Ketones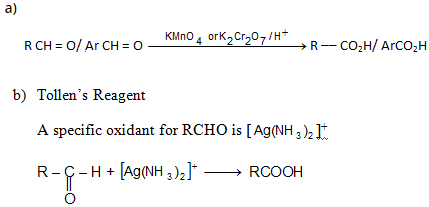
Tollen’s test chiefly used for the detection of aldehydes.
Tollen’s reagent doesnot attack carbon-carbon double bonds.
c) Strong Oxidants: Ketones resist mild oxidation, but with strong oxidants at high temperature they undergo cleavage of C – C bonds on either sides of the carbonyl group.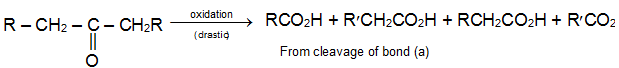
d) Haloform Reaction
CH3COR are readily oxidised by NaOI (NaOH + I2) to iodoform, CHI3, and RCO2Na
Example: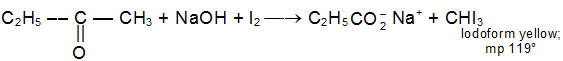
- Reduction:
a) Reduction to alcohols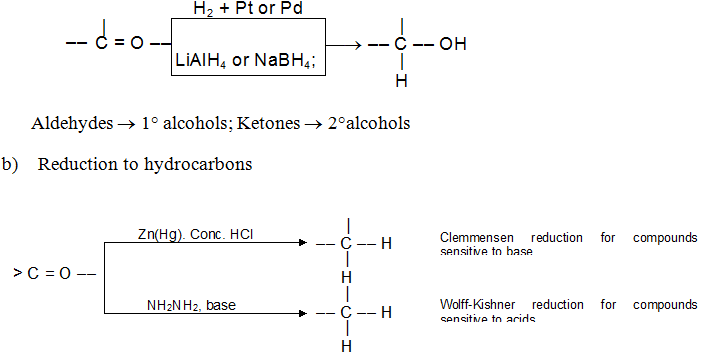
Carboxylic Acids:
Carboxylic Acids | Common Names |
HCOOH | Formic acid |
CH3COOH | Acetic acid |
CH3–CH2–COOH | Propionic acid |
CH3(CH2)COOH | Butyric acid |
CH3(CH2)3COOH | Valeric acid |
CH3(CH2)14COOH | Palmitic acid |
CH3(CH2)16COOH | Stearic Acid |
Physical Properties of Carboxylic Acids
- The first three acids are colourless, pungent smelling liquids.
- First four members are miscible in water due the intermolecular hydrogen bonding whereas higher members are miscible in non – polar solvents like ether.
- Benzene or ethanol but immiscible in water due to the increase in the size of lyophobic alkyl chain.
- The b.p. of carboxylic acids are higher than alcohols because carboxylic acids exist as dimers due to the presence of intermolecular H-bonding
- Increase in the number of Halogen atoms on a-position increases the acidity, eg.
CCl3COOH > CHCl2COOH > ClCH2COOH > CH3COOH - Increase in the distance of Halogen from COOH decreases the acidity e.g
CH3 – CH2 – CH(Cl) – COOH > CH3 – CH(Cl) – CH2 – COOH > CH2 – CH2 – CH2 – COOH - Increase in the electro negativity of halogen increases the acidity.
FCH2COOH > BrCH2COOH > ICH2COOH
Methods of Preparations of Carboxylic Acids
a. Oxidation of Aldehydes & Ketones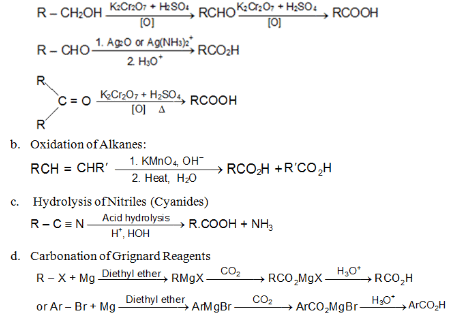
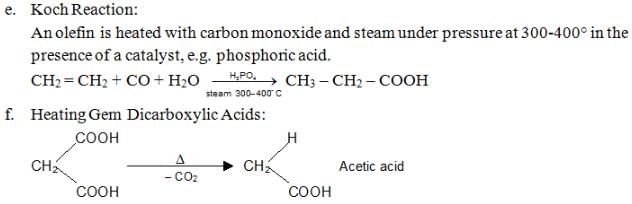
Chemical Reactions of Carboxylic Acids
a. Salt formation:
2CH3COOH + 2Na → 2CH3COO–Na+ + H2
CH3COOH + NaOH → CH3COO–Na+ + H2O
CH3COOH + NaHCO3 → CH3COO–Na+ + H2O + CO2
b. Conversion into Acid Chlorides: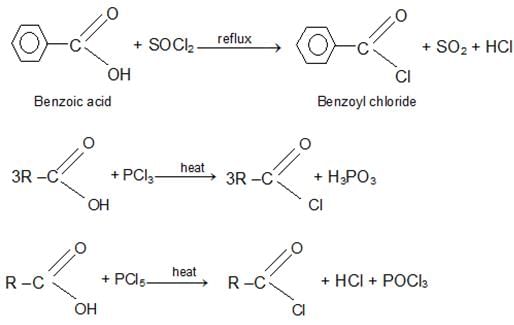
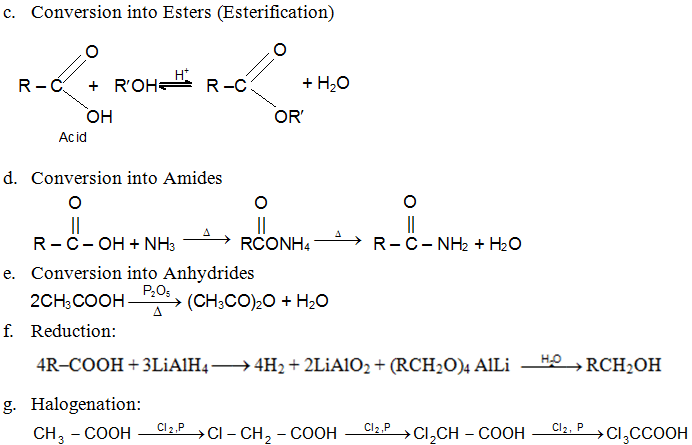
Esters
a) Transesterification :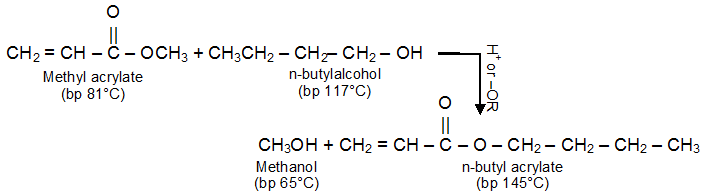
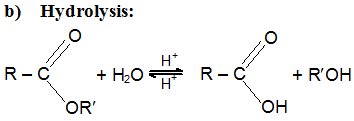
c) Reduction: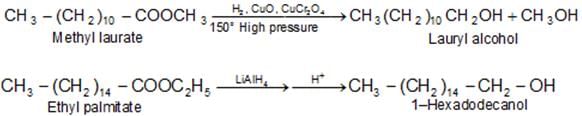
Acid Chlorides: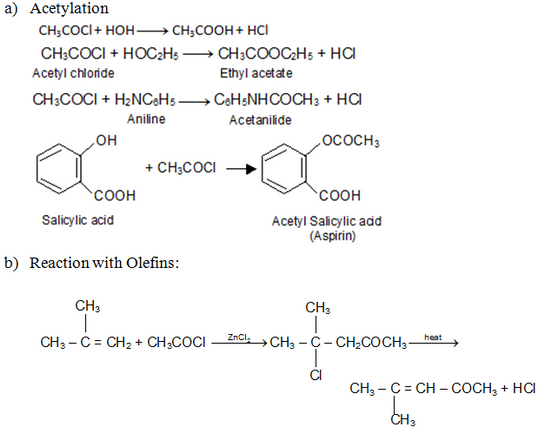
c) Conversion of Acid Chlorides into Acid Derivatives: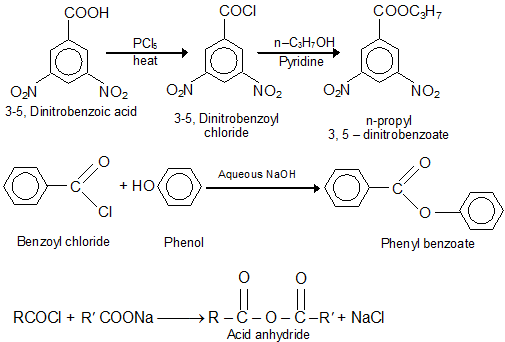
Amides
a. Hydrolysis: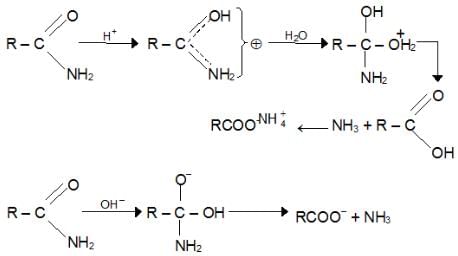
b. Acidic Character of Amides:
2RCONH2 + HgO → (RCONH)2Hg + H2O
c. Basic Character of Amides:
Amides are very feebly basic and form unstable salts with strong inorganic acids. e.g. RCONH2HCl. The structure of these salts may be I or II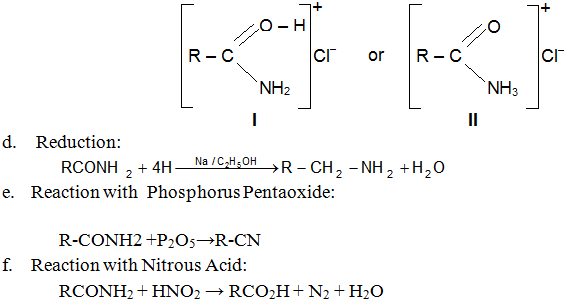
FAQs on Aldehydes, Ketones & Carboxylic Acids Class 12 Notes Chemistry Chapter 8
| 1. What are the common properties of aldehydes, ketones, and carboxylic acids? |  |
| 2. How are aldehydes and ketones named? |  |
| 3. What are some common uses of carboxylic acids? |  |
| 4. How do aldehydes and ketones react with nucleophiles? |  |
| 5. What is the difference between a primary, secondary, and tertiary alcohol? |  |
















