Alkenes: Nomenclature, Properties & Preparation | Chemistry Class 11 - NEET PDF Download
Alkenes
1. Introduction
Alkenes are hydrocarbons with carbon-carbon double bonds, Alkenes are sometimes called olefins, a term derived from olefinic gas, meaning "oil forming gas". Alkenes are among the most important industrial compound and many alkenes are also found in plants and animals. Ethylene is the largest-volume industrial organic compound, used to make polyethylene and a variety of other industrial and consumer chemicals.
2. Structure and bonding in Alkenes:
- Alkenes are unsaturated hydrocarbons having at least one double bond.
- They are represented by general Formula (G.F.) CnH2n (one double bond)
- In Ethene, C = C bond length is 1.34 Å.
- Its bond energy is 146 kcal.mol-1
- The hybridization of (C = C) alkenic carbon is sp2
- The π e- cloud is present above and below the plane of s-bonded skeleton.
- They are also known as olefins since ethene, the first member of the homologous series forms oily liquid substance when treated with halogens.
- Compounds may exist as conjugated polyenes or as cumulated polyenes or as isolated polyenes.
Note : That angle a > b since repulsion due to p electrons (double bond - single bond repulsion > single bond single bond repulsion according to VSEPR theory.
IUPAC System of Alkenes
- The longest carbon chain containing the carbon-carbon double bond is selected as the parent alkene.
- The suffix ‘ane’ of the alkane is replaced by ‘ene’. If a double bond occurs twice or thrice in the parent chain the alkene is called diene or triene respectively.
- The position of double bonds or side chains indicated by numbers 1, 2, 3 etc.
- The longest chain is numbered from that end, which gives the lowest number to the carbon atom of the double bond and written just before the suffix ‘ene’. If while numbering the chain the double bond gets the same number from either side the carbon chain is numbered in such a manner that the substituent gets the lowest number.
- In case there are two or more double bonds, the lowest sum rule should be followed.
- The name and position of other groups (substituents) is indicated by prefixes.
Ex.1 Write IUPAC names of:
(a) (b)
Ans. (a) 2, 3-Dimethylcyclohexene
(b) 1-(2-butenyl) cyclohex -1-ene
Ex.2 Give the structure for each of the following:
(a) 4-Methyl-1, 3-hexadiene
(b) 1-Isopropenylcyclopentene
Ans. (a) (b)
3. Physical Properties of Alkenes / Hydrocarbons:
| Physical properties | Homologous series | Isomers |
1. | Physical state | C1 - C3 gases, C4 - C20 liquids, >C20 : solids |
|
2. | Dipole moment (n) |
| cis > trans |
3. | Polarity | - | cis > trans (for Cab = Cab type of alkenes) |
4. | Melting point | decreases as the homologous series increase. | trans > cis (due to more packing capacity) |
5. | Boiling point | increases as the homologous series increase. | cis > trans # branching decreases B.P. Polarity increases, boiling point increases |
6. | Solubility | Practically insoluble in water but fairly soluble in non-polar solvents like benzene-petroleum ether, etc. | cis > trans Polarity increases, solubility in polar solvents increases. |
7. | Stability |
| trans > cis |
4. Laboratory test of Alkenes:
5. Methods of preparation of alkenes:
(i) By Partial Reduction of Alkynes:
(a) By Catalytic Hydrogenation of Alkynes in presence of poisoned catalyst(A Syn Addition of Hydrogen : Synthesis of cis-Alkenes: This is performed by:
(i) Lindlar's catalyst : Metallic palladium deposited on calcium carbonate with lead acetate and quinoline.
(ii) P-2 catalyst (Ni2B: nickel boride)
General Reaction: R - C º C - R
Mechanism of hydrogenation :
Steps : The reactant alkyne molecules and hydrogen molecules get adsorbed at the surface of metal catalyst. It is chemical adsorption (chemisorption).
In this state, the reactants lie very close to each other and so the hydrogen atoms start forming bonds with carbon. Two hydrogen atoms are added to two triply bonded carbon atom from the same side of p bond and a cis or syn addition product is formed. The product alkene now escapes away from the surface of the catalyst. Quinoline occupies the metal surface inhibiting further reduction to alkanes. Quinoline therefore is called catalyst poison and palladium is called deactivated catalyst or poisoned catalyst.
e.g.
(b) Birch Reduction : (Anti Addition of Hydrogen : Synthesis of trans-Alkenes)
General Reaction:
Mechanism : Reagents Na(or Li, K) + liq NH3 → Na+ + e– (solvated electron)
Note : This process of reduction is not eligible when terminal alkenes are taken. (R - C º CH) because terminal alkenes form sodium salt with Na metal.
CH3 - C = CH + Na / NH3 → CH3 - CH = C- Na+ + [H]+
Ex.3 Identify the reagent for following synthesis.
Ans. H2 / Lindiar's catalyst.
Ex.4 Identify the products in the following reaction :
Ans. 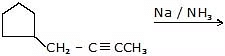
(ii) By Dehydrohalogenation of Vicinal Di-Halides:
There are two types of dihalides namely gem (or geminal) dihalides in which the two halogen atoms are attached to the same carbon atom and vicinal dihalides in which the two halogen atoms are attached to the adjacent carbon atoms.
Dehalogenation of vicinal dihalides can be effective either by NaI in acetone or zinc in presence of acetic acid or ethanol.
General Reaction:
(i)
(ii) CH3 - CHBr - CH2Br CH3 - CH = CH2
Mech.
With NaI in acetone :
It involves an anti elimination of halogen atoms.
Remarks:
(1) Both are E2 elimination.
(2) Both are stereospecific anti elimination.
(iii) Dehydro-Halogenation of Alky Halides:
Dehydro - halogenation is the elimination of a hydrogen and a halogen from an alkyl halide to form an alkene.
Dehydro halogenation can take place by E1 and E2 mechanism.
(i) Hot alcoholic solution of KOH EtO- / EtOH.
(ii) NaNH2.
(iii) t-BuO-K+ in t-BuOH.
(i) Dehydrohalogenation by the E2 mechanism: Second-order elimination is a reliable synthetic reaction, especially if the alkyl halide is a poor SN2 substrate. E2 dehydrohalogenation takes place in one step, in which a strong base abstracts a proton from one carbon atoms as the leaving group leaves the adjacent carbon.
General reaction :
e.g.
Here β - H is eliminated by base hence called β-elimination following Saytzeff rule.
i.e, (Highly substituted alkene is major product). It also involves an anti elimination of HX.
e.g.
e.g.
e.g.
e.g.
(ii) Formation of the Hoffmann product:
Bulky bases can also accomplish dehydro halogenation that do not follow the saytzeff rule. Due to steric hindrance, a bulky base abstracts the proton that leads to the most highly substituted alkene. In these cases, it abstracts a less hindered proton, often the one that leads to formation of the least highly substituted product, called the Hoffmann product.
Stereospecific E2 reactions:
The E2 is stereospecific because it normally goes through an anti and coplanar transition state. The products are alkene, and different diastereomers of starting materials commonly give different diastereomers of alkenes.
Ex.5 What alkyl halide would yield each of the following pure alkene on reaction with alcoholic KOH ?
(i)
(ii) CH3 - CH2 - CH2 - CH = CH2
Ans. (i)
(ii) CH3CH2CH2CH2 CH2Cl
(iii)
Ex.6 What are the various product due to loss of HBr from
Ans.
(iv) Dehydration of Alcohols
Alcohols when heated in presence of following reagents undergo loss of water molecule and form alkenes. The elimination is β elimination.
(i) H2SO4 / 160ºC
(ii) H3PO4 / Δ
(iii) P2O5 / Δ
(iv) Al2O3 / 350ºC undergo loss of water molecule and form alkenes.
General Reaction: RCHCH2OH R - CH = CH2 H2O
e.g.
(V) By Pyrolysis of Esters:
Thermal cleavage of an ester involves formation of a six membered ring in the transition state leading to the elimination of an acid leaving behind an alkene.
As a direct consequence of cyclic transition state, both the leaving groups namely proton and carboxylate ion are eliminated from the cis position. This is an example of syn elimination.
(VI) By Hoffmann Elimination Method:
Alkenes can be prepared by heating quaternary ammonium hydroxide under reduced pressure at a temperature between 100ºC and 200ºC.
Less substituted alkenes are formed as major product in this case, which are defined as Hoffmann alkenes.
(VII) By Wittig Reaction:
The aldehydes and ketones are converted into alkenes by using a special class of compounds called phosphorus ylides, also called Wittig reagents.
The Triphenyl group of phosphorane has a strong tendency to pull oxygen atom of the aldehyde or ketone via a cyclic transition state forming an alkene.
(R, R', R" and R"' may be hydrogen or any alkyl group)
e.g.
Ex.7 Complete the following reaction :
Ans.
Ex.8 Identify the (X), (Y), and (Z) in the following reactions
(i) PhCH2Br CH3 - - CH3
(X)
(ii) CH3I PhCOCH3 (Y)
(iii) PhCH2Br PhCH = CHCHO (Z)
Ans. (X) = Ph - CH = C(CH3)2
(Y) = Ph - C(CH3) = CH2
(Z) = Ph - CH = CH - CH = CH - Ph
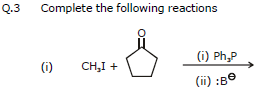
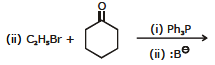
6. Chemical reactions of alkenes:
(I) Catalytic Hydrogenation Of Alkenes : Heterogenous Hydrogenation)
Hydrogenation : The function of catalyst
Hydrogenation of a alkene is exothermic reaction (DHº = - 120 kJ mol-1)
R - CH = CH - R H2 R - CH2 - CH2 - R heat
As a consequence ,both hydrogen atoms usually add from the same side of the molecule. This mode of addition is called a syn addition.
Hydrogenation of an alkene is formally a reduction, with addition of H2 across the double bond to give an alkane.
The process usually requires a catalyst containing Pt, Pd or Ni.
e.g. CH3 - CH - = CH - CH3 H2 CH3 - CH2 - CH2 - CH3
e.g.
Ex.9 Complete the following reactions :
CH3CH = CH2 + H2 ?
Sol. CH3CH = CH2 + H2 CH3CH2CH3
(II) Electrophilic Addition Reactions :
Mechanism:
Step 1 : Attack of the electrophile on p bond forms a carbocation.
Step 2 : Attack by a nucleophile gives the product of addition
(i) Acid-Catalyzed Hydration of Alkenes
Alkenes add water in the presence of an acid catalyst to yield alcohols. The addition takes place with Markovnikov's rule. The reaction is reversible, and the mechanism for the acid-catalyzed hydration of an alkene is simply the reverse of that for the dehydration of an alcohol.
The carbocation intermediate may rearrange if a more stable carbocation is possible by hydride or alkanide migration. Thus, a mixture of isomeric alcohol products may result.
General Reaction:
Mech.
Step 1. : Protonation of the double bond forms a carbocation-
Step 2 : Nucleophilic attack by water-
Step 3 : Deprotonation to the alcohol-
e.g.
e.g.
Ex.10 Identify the product in following reaction:
Ans.
(ii) (a) Oxymercuration - Demercuration:
Alkenes react with mercuric acetate in a mixture of water and tetrahydrofuran (THF) to produce (hydroxyalkyl) mercury compounds. These can be reduced to alcohols with sodium borohydride and water :
Oxymercuration:
General Reaction:
In the oxymercuration step, water and mercuric acetate add to the double bond; in the demercuration step, sodium borohydride reduces the acetoxymercury group and replaces in with hydrogen. Then net addition of H -and -OH takes place with Markovnikov's rule and generally takes place without the complication of rearrangements.
e.g.
(b) Alkoxymercuration - demercuration:
General reaction:
e.g.
Ex.11 Supply the structures for (X) and (Y) in the following two - step reaction: :
C3H7CH = CH2
Sol. (X) = C3H7CH(OH)CH2-HgOAC (Y) = C3H7CH(OH)CH3
(An organomercurial alcohol)
Ex.12 Identify final product in the following :
(a)
(b)
Ans. (a) (b)
(iii) Hydroboration-oxidation (Syn Addition):
General Reaction:
An alkene reacts with BH3 : THF of diborane to produce an alkylborane. Oxidation and hydrolysis of the alkylborane with hydrogen peroxide and base yields an alcohol.
e.g.
Oxidation:
In the first step, boron and hydrogen undergo syn addition to the alkene in the second step, treatment with hydrogen peroxide and base replaces the boron with -OH with retention of configuration. The net addition of -H and -OH occurs with anti Markovnikov regioselectivity and syn stereoselectivity. Hydroboration -oxidation therefore, serves as a useful regiochemical complement to oxymercuration demercuration.
e.g.
e.g.
(i) Hydration with dil. H2SO4 proceeds via carbocation rearrangement.
(ii) Hydration with Hg(OAc)2, H2O, following by NaBH4 proceeds via Markonikov's rule.
(ii) Hydration with (BH3)2 followed by H2O2 / OH- proceeds via Anti Markonikov's rule.
(iv) Addition of hydrogen halides:
General Reaction:
Note:(1) Anti-Markovnikov addition is valid only for HBr in presence of peroxide and light only.
(2) HF, HCl and HI give only polar addition and give Markovnikov product only.
e.g.
e.g.
Ex.13 Predict the major products of the following reactions and propose mechanism to support your predictions.
(A)
(B) CH3 - CH2 - O - O - CH2 - CH3
(C)
Sol. (A)
(B)
(C)
Ex.14 Identify the products in the following reactions :
(a) F3C - CH = CH2 HCl
(b) O2N - CH = CH2 HCl
(c) CH3O - CH = CH2 HCl
(d) PhCH = CHCH3 HCl
(e)
Q.6 Give the products of the following reactions : -
Q.7 Give the reactant (alkene) of the following products.
(v) Addition of halogen
Halogen add to alkenes to form vicinal dihalides.
General Reaction:
(X2 = Cl2, Br2)
The nucleophile attacks the electrophilic nucleus of one halogen atom, and the other halogen serves as the leaving group, departing as halide ion. Many reactions fit this general pattern.
Note : (i) F2 is not added because F+ is never generated. Morever reaction is explosive giving CO2 & H2O.
(ii) I2 is not added because reaction is reversible with equilibrium in backward direction.
(iii) Reaction with bromine is basis for test of alkenes.
(iv) Halogen addition is stereospecific anti addition.
(v) Halogens can also be added in presence of sun light and give free radical addition.
(Reactivity of halogen addition in sunlight is F2 (explosive) > Cl2 > Br2 > I2)
Mech.
Step-1 Formation of a halonium ion
Step-2 Opening of the halonium ion
X. -attacks from the back side of halonium ion.
e.g.
e.g.
(vi) Addition of dihydrogen
Alkanes are formed on the addition of one molecule of dihydrogen to alkenes in the presence of different types of catalysts such as palladium, nickel or platinum.
(vii) Ozonolysis
Ozonolysis refers to the organic chemical reaction where ozone is employed to cleave the unsaturated bonds of alkenes, alkynes, and azo compounds (compounds with the functional diazenyl functional group). It is an organic redox reaction.
- Oxidation of alkenes with the help of ozone can give alcohols, aldehydes, ketones, or carboxylic acids.
- Alkynes undergo ozonolysis to give acid anhydrides or diketones. If water is present in the reaction, the acid anhydride undergoes hydrolysis to yield two carboxylic acids.
- Ozonolysis of elastomers is also known as ozone cracking. Trace amounts of ozone gas in the atmosphere cuts the double bonds in elastomers.
- For azo compounds, the ozonolysis yields nitrosamines.
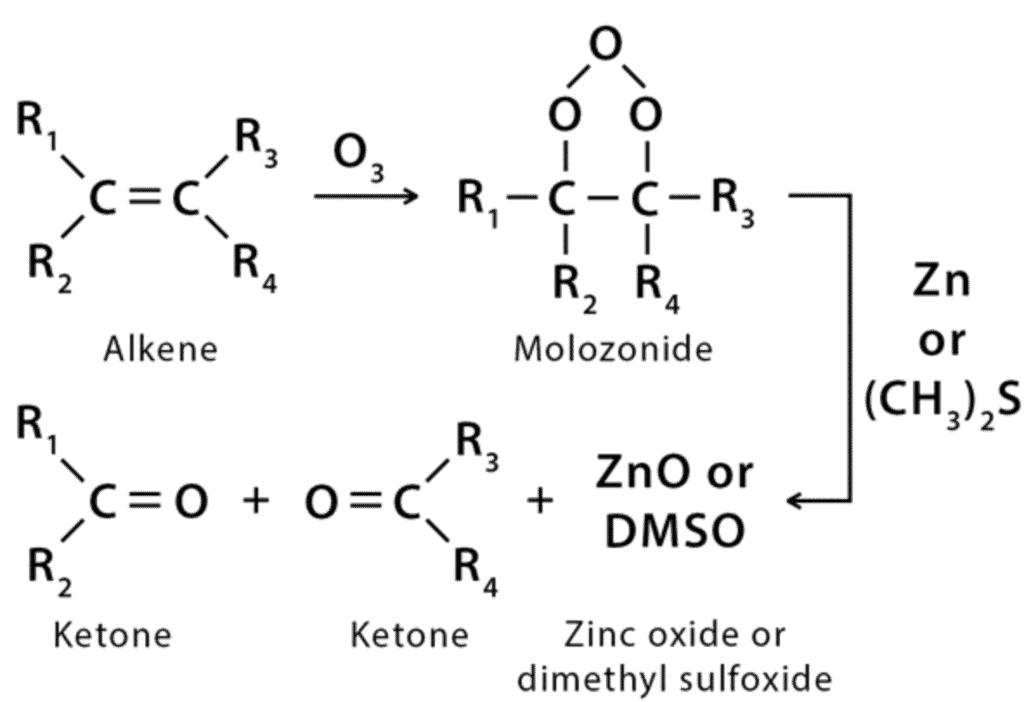
(viii) Polymerisation
Polythene is obtained by the combination of large number of ethene molecules at high
temperature, high pressure and in the presence of a catalyst. The large molecules thus obtained are called polymers. This reaction is known as polymerisation. The simple compounds from which polymers are made are called monomers. Other alkenes also undergo polymerisation.
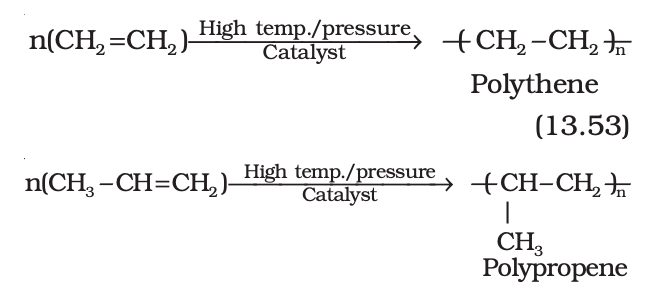
Uses of Polymers:
- Polymers are used for the manufacture of plastic bags, squeeze bottles, refrigerator dishes, toys, pipes, radio and T.V. cabinets etc.
- Polypropene is used for the manufacture of milk crates, plastic buckets and other moulded articles. Though these materials have now become common, excessive use of polythene and polypropylene is a matter of great concern for all of us.
|
114 videos|263 docs|74 tests
|
FAQs on Alkenes: Nomenclature, Properties & Preparation - Chemistry Class 11 - NEET
| 1. What is the IUPAC nomenclature for alkenes? |  |
| 2. What are the properties of alkenes? |  |
| 3. How can alkenes be prepared? |  |
| 4. What are the physical properties of alkenes? |  |
| 5. How do the properties of alkenes differ from alkanes? |  |





















