Allotropes of Carbon & Hydrocarbons | Science Class 10 PDF Download
| Table of contents |

|
| What are Allotropes of Carbon? |

|
| Diamond |

|
| Graphite |

|
| Fullerene |

|
| What are Hydrocarbons? |

|
Carbon is found in various forms in nature, each with distinct physical properties. While diamond and graphite consist of carbon atoms, the way these atoms bond together distinguishes one form from the other.
What are Allotropes of Carbon?
The phenomenon of the existence of allotropic forms of an element is called allotropy. Allotropes are the different forms of the same element having different physical properties but almost similar chemical properties due to different arrangements of atoms.
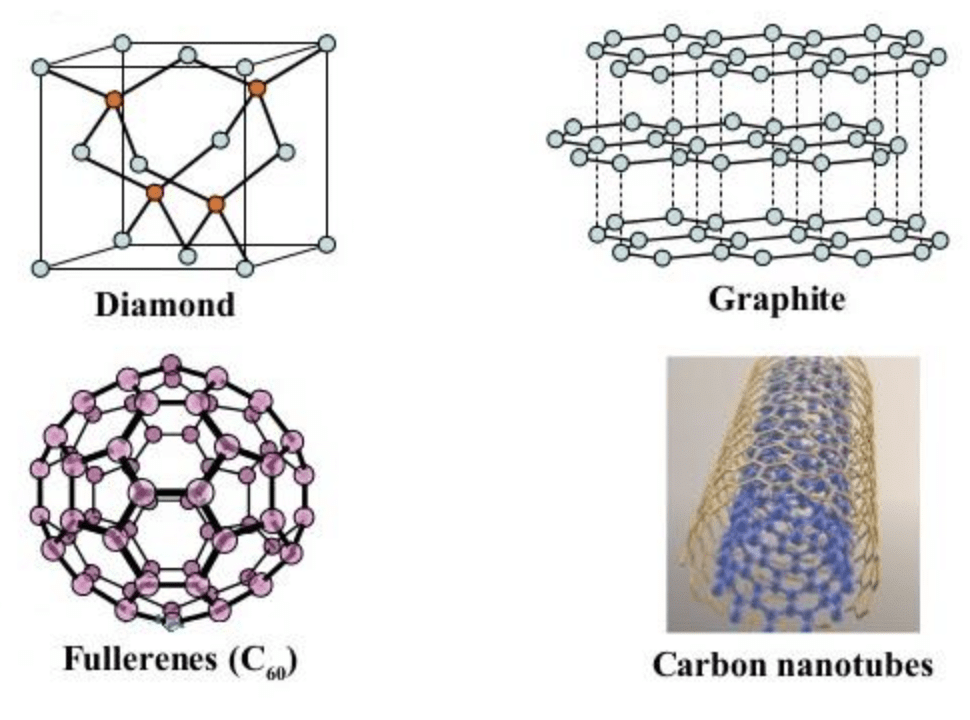 Allotropes of Carbon
Allotropes of Carbon
The allotropes of carbon can be categorized into two:
- Amorphous Carbon Allotropes: Amorphous carbon allotropes refer to forms of carbon that lack a well-defined crystal structure. These allotropes are characterized by a disordered arrangement of carbon atoms, resulting in a non-crystalline or glassy structure. Unlike crystalline carbon allotropes, amorphous carbon does not have a repeating pattern of atoms.
Common examples of amorphous carbon allotropes include: Carbon Black, Activated Carbon, Charcoal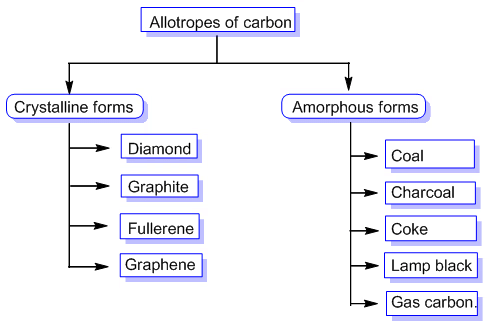
- Crystalline Carbon Allotropes: Crystalline carbon allotropes refer to forms of carbon that have a well-defined crystal structure. These allotropes are characterized by a regular arrangement of carbon atoms, forming distinct crystal lattices. Crystalline carbon exhibits different properties and structures depending on the arrangement of atoms.
The most well-known crystalline carbon allotropes include: Diamond, Graphite, Fullerenes, Carbon Nanotubes
Let us now take a look into the more widely known allotropes of carbon:
Diamond
Diamond is a crystalline allotrope of carbon. Its atomic symbol & empirical formula is 'C'
Structure of Diamond
In a diamond, each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement. This tetrahedral arrangement of carbon atoms gives a rigid, three-dimensional structure to diamonds.
Because of the rigid structure of diamonds:
- It is a very hard crystalline structure.
- It has a high melting point.
- It is a non-conductor of heat and electricity.
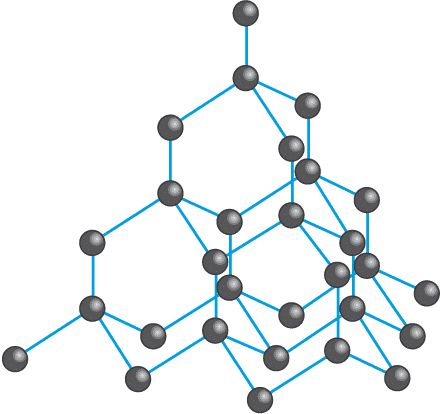 Structure of Diamond
Structure of Diamond
Properties of Diamond
- Pure diamond is a transparent and colourless solid.
- Polished diamond sparkles brightly because it reflects most of the light.
Uses of Diamond
- A saw fitted with a diamond is used for sawing marbles.
- A chip of diamond is used for glass cutting.
- Diamonds are used for making dice for drawing very thin wires of harder metals.
- Diamonds are also used for making high-precision tools for use in surgery such as for the removal of the cataract.
- Diamonds are used for making precision thermometers and protective windows for space crafts.
Graphite
Graphite is also known as black lead, it marks paper black. The name graphite has been taken from the Greek word ''graphein" (which means to write) in reference to its uses as 'lead' in lead pencils.
Structure of Graphite
- Graphite is an opaque and dark grey solid.
- In a crystal of graphite, the carbon atoms are arranged in hexagonal patterns in parallel planes.
- In a layer of graphite, each carbon atom is strongly bonded to three carbon atoms by covalent bonds. Thus, one valence electron of each carbon atom is free in every layer of graphite crystal.
- The free electron makes graphite a good conductor of electricity.
- Each layer is bonded to the adjacent layers by weak Vander Waals forces. As a result, each layer can easily slide over the other.
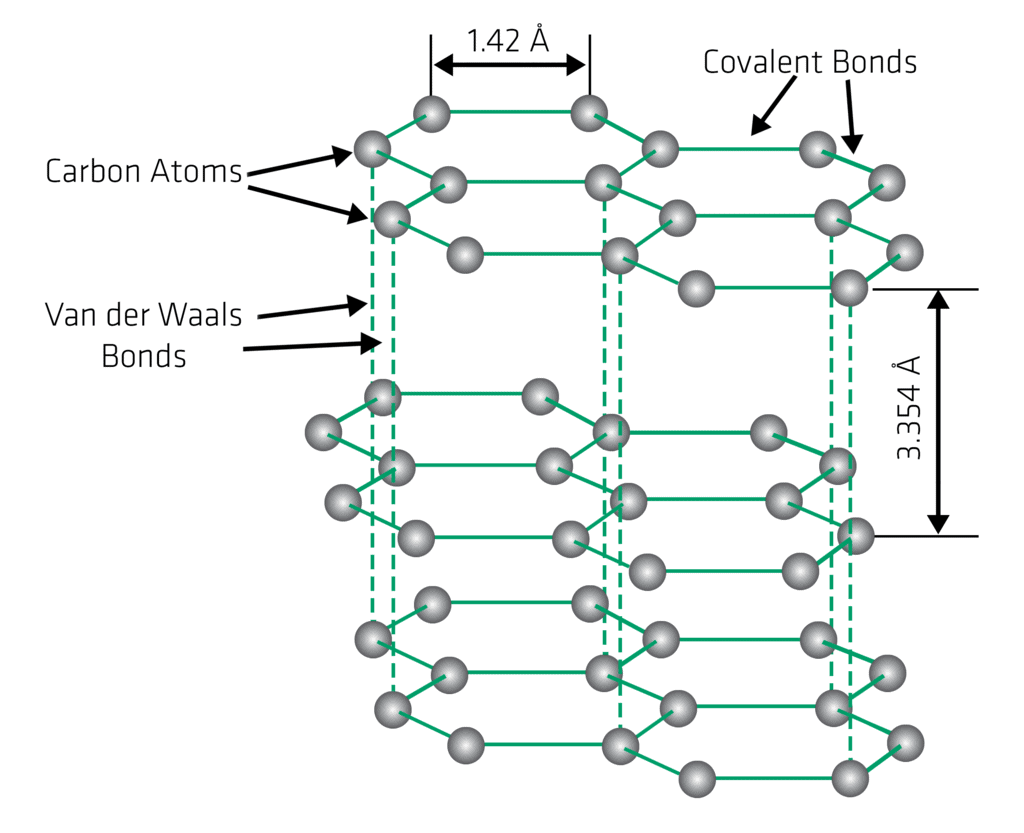 Structure of Graphite
Structure of Graphite
Properties of Graphite
- Graphite is greyish-black, opaque material having metallic (shiny) lustre.
- It is soft and slippery.
- Graphite is lighter than diamond.
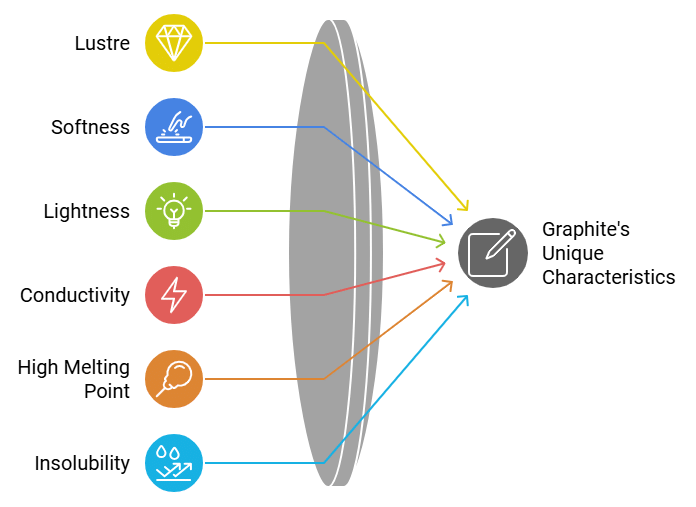
- Graphite is a good conductor of heat and electricity.
- Graphite has a very high melting point.
- Graphite is insoluble in all common solvents.
Uses of Graphite
- For making electrodes in dry cells and electric arc furnaces.
- Graphite is a good dry lubricant for those parts of machines where grease and oil cannot be used.
- For making crucibles for melting metals.
- For manufacturing lead pencils.
- Graphite is used as a neutron moderator in nuclear reactors.
- For the manufacture of gramophone records and in electrotyping.
- For the manufacture of an artificial diamond.
- For the manufacture of black dye and paints.
Fullerene
Fullerene was discovered in 1985 by Robert F. Curl Jr, Harold Kroto and Richard E. Smalley.
- This molecule containing sixty atoms of carbon has been named Buckminster Fullerene. Fullerenes have been named after American architect and engineer R. Buckminster-fuller whose geodesic domes follow similar building principles.
- Types of fullerene: C60, C70, C74 and C78
But C60 is the most stable and most studied form of fullerenes.
Structure of Fullerene
- Buckminsterfullerene molecule (C60) is nearly spherical.
- It consists of 12 pentagonal faces and 20 hexagonal faces giving it 60 corners. Thus, Buckminsterfullerene has a hollow, cage-like structure.
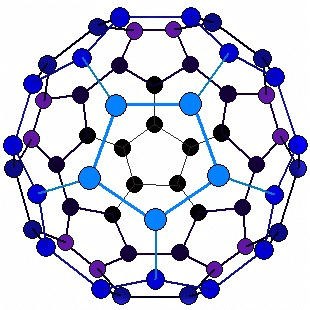 Structure of Fullerene
Structure of Fullerene - In the figure, it has a ball-like structure whose molecules contain C atoms.
Preparation of Fullerene
- By electrically heating a graphite rod in the atmosphere of helium.
- By vaporising graphite using a laser.
Properties of Fullerene
- Fullerene is soluble in benzene and forms a deep violet colour solution.
- Crystalline fullerene has semiconductor properties.
- Compounds of fullerene with alkali metals are called fullerides and they are superconductors.
Uses of Fullerene
- As a superconductor.
- As a semiconductor.
- As a lubricant and catalyst.
- As fibres to reinforce plastics.
What are Hydrocarbons?
Compounds formed by the combination of carbon and hydrogen atoms only are known as hydrocarbons.
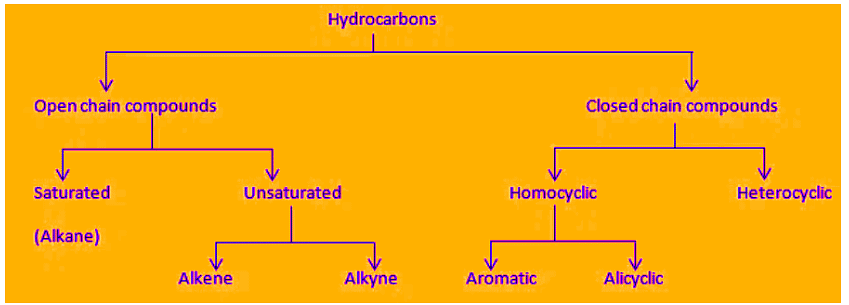 Classification of Hydrocarbons
Classification of Hydrocarbons
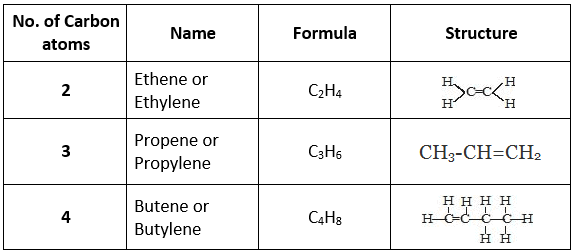
Saturated Hydrocarbon
- The hydrocarbons which contain only single carbon-carbon covalent bonds are called saturated hydrocarbons.
- They are also called alkanes.
- The general formula for alkanes is CnH2n+2 where 'n' is the number of carbon atoms.
 Some examples of saturated hydrocarbons
Some examples of saturated hydrocarbons
Unsaturated Hydrocarbons
- The hydrocarbon in which two carbon atoms are bonded to each other by a double (=) or a triple (≡) bond is called an unsaturated hydrocarbon.
- Unsaturated hydrocarbons are of two types:
(a) Alkenes (>C=C<): The hydrocarbons in which the two carbon atoms are bonded by a double bond are called alkenes.
Their general formula is CnH2n where "n" is the number of carbon atoms.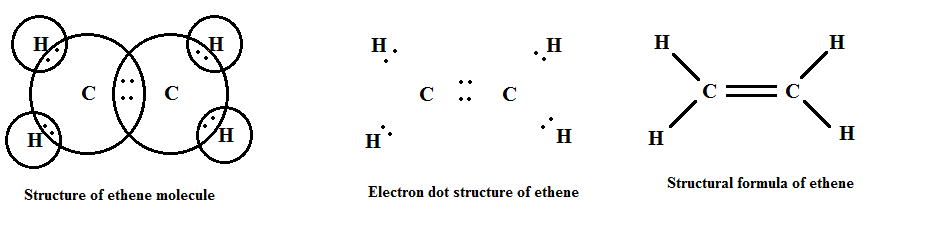
 Some examples of alkenes
Some examples of alkenes
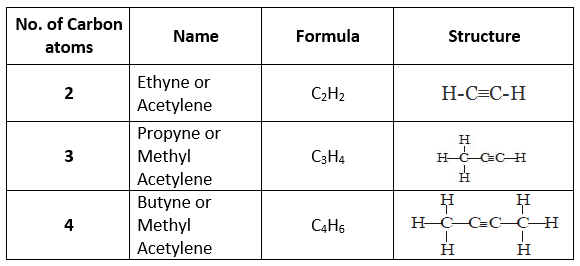
(b) Alkyne (-C≡C-): The hydrocarbons in which two carbon atoms are bonded by a triple bond are called alkynes.
Their general formula is CnH2n-2 where 'n' is the number of carbon atoms.
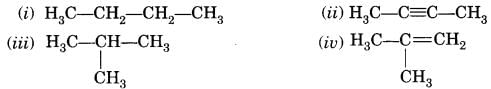
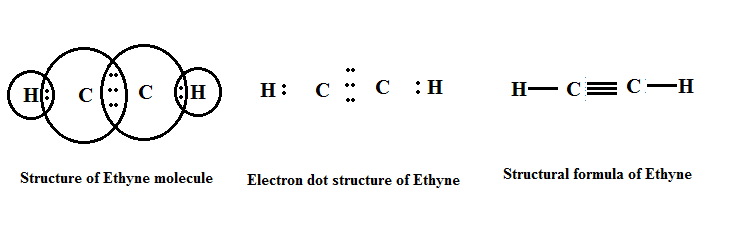
|
80 videos|569 docs|80 tests
|
FAQs on Allotropes of Carbon & Hydrocarbons - Science Class 10
| 1. What are the main allotropes of carbon? |  |
| 2. How do the physical properties of diamond and graphite differ? |  |
| 3. What are hydrocarbons and how are they classified? |  |
| 4. What are some common uses of hydrocarbons? |  |
| 5. How do the environmental impacts of hydrocarbons affect their usage? |  |





















