Aromatic Hydrocarbons: Nomenclature, Properties, Reactions, Uses & Polycyclic Aromatic Hydrocarbons | Chemistry Class 11 - NEET PDF Download
What are Aromatic Hydrocarbons?
Aromatic Hydrocarbons are circularly structured organic compounds that contain sigma bonds along with delocalized pi electrons. They are also referred to as arenes or aryl hydrocarbons.
Aromatic Hydrocarbons, often called arenes, are a type of hydrocarbon characterized by sigma bonds and delocalized pi electrons between carbon atoms arranged in a ring formation, such as in benzene. The term "aromatic" is used because these compounds typically emit a pleasant fragrance.
IUPAC Nomenclature of Aromatic Hydrocarbons
- In the past, many compounds with identical structural formulas were given different names based on their regions of origin, leading to confusion. To address this issue, a unified naming system with standardized rules was developed by the International Union for Pure and Applied Chemistry (IUPAC) for these compounds.
- The IUPAC nomenclature provides clear guidelines for naming aromatic hydrocarbons, ensuring consistency and clarity in chemical communication.
Rules for Naming Aromatic Hydrocarbons
- When naming substituted aromatic compounds, the names of substituents are added as prefixes to the names of aromatic compounds. For example, a benzene ring with one nitro group is called nitrobenzene.
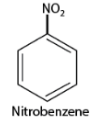
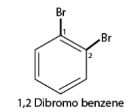
- If there are multiple similar substituent groups in the ring, Greek numerical prefixes like di, tri, and tetra are used to indicate the number of similar groups attached. For instance, 1,2-dibromobenzene denotes two bromo groups on adjacent carbon atoms of the benzene ring.
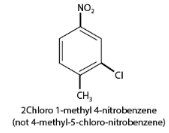
- When different substituent groups are present, the substituent of the base compound is assigned the number one. Numbering is done to give the next substituent the lowest number, with names listed in alphabetical order. For example, in a ring with chloro and nitro groups, the chloro group is identified first, followed by the nitro group.
- For compounds with multiple substituents, prefixes like ortho (o), meta (m), and para (p) are used to indicate their positions: 1,2-; 1,3-; and 1,4- respectively. For instance, 1,2-dibromobenzene can also be referred to as o-dibromobenzene.
- If an alkane with a functional group is attached to an aromatic compound, the aromatic part is treated as a substituent rather than the main compound. For example, a benzene ring attached to an alkane with a functional group is called phenyl, indicated by Ph-.
Characteristics of Aromatic Hydrocarbons
Benzene was the first compound recognized as an aromatic hydrocarbon, and it remains the most complex aryl hydrocarbon. In a benzene ring, each carbon atom is bonded to:
- Two other carbon atoms by sigma bonds,
- One hydrogen atom by a sigma bond, and
- One neighboring carbon atom with a double bond, where the pi electrons are delocalized.
General Characteristics
- Aromatic hydrocarbons exhibit aromaticity, which enhances their stability due to resonance.
- These molecules typically have a hydrogen-to-carbon ratio of 1:1, which is characteristic of aromatic compounds.
- When combusted, aromatic hydrocarbons produce a strong, yellow, and sooty flame.
- They primarily undergo electrophilic substitutions and nucleophilic aromatic substitution reactions.
- Aromatic hydrocarbons can be either monocyclic or polycyclic.
Reactions of Aromatic Hydrocarbons
Many organic chemical reactions involve the use of aromatic hydrocarbons as the primary reactant. Some such reactions are listed in this subsection along with a brief description of each of these reactions.
1. Aromatic Substitution Reactions
Aromatic substitution reactions involve replacing a substituent on the aromatic hydrocarbon ring, typically a hydrogen atom, with a different substituent group. There are several types of aromatic substitution reactions, including:
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reactions
- Radical nucleophilic aromatic substitution reactions
One example of an aromatic substitution reaction is the electrophilic substitution observed in the nitration of salicylic acid, where a nitro group (NO2) is introduced onto the aromatic ring.
2. Coupling Reactions
Coupling reactions involve joining two radical parts together with the help of a metal catalyst. When aromatic hydrocarbons undergo coupling reactions, different types of bonds can be formed.
- Carbon-carbon bonds can be created by coupling arenes, leading to the formation of products like vinyl arenes and alkyl arenes.
- These reactions can also produce carbon-oxygen bonds, resulting in the formation of aryloxy compounds.
- Carbon-nitrogen bonds can be formed in coupling reactions, leading to products such as aniline.
One example of a coupling reaction involving aromatic hydrocarbons is the arylation of arenes or aromatic compounds. In this reaction, palladium(II) acetate is used as the catalyst, and DMA refers to dimethylacetamide.
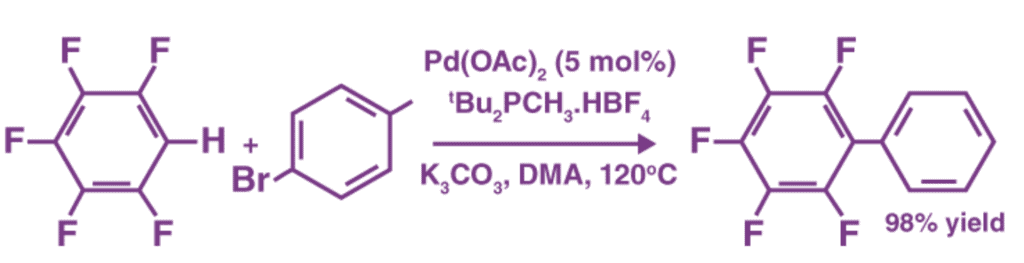
3. Hydrogenation Reactions
- The hydrogenation reactions involving arenes generally lead to the formation of saturated rings. An example of such a reaction is the reduction of 1-naphthol into a mixture containing different isomers of decalin-ol.
- Another example of such a reaction is the hydrogenation reaction of resorcinol with the help of spongy nickel (also referred to as Raney nickel) and aqueous NaOH. This reaction proceeds via the formation of an enolate, and the successive alkylation of this enolate (with methyl iodide) to yield 2-methyl-1,3-cyclohexanedione.
Applications of Aromatic Hydrocarbons
Aromatic hydrocarbons are commonly used in various biological and synthetic processes. Here are some of their applications:
- Chlorophyll: Chlorophyll contains a porphyrin ring with aromatic properties, which is essential for photosynthesis and food production in plants.
- Nucleic Acids and Amino Acids: Some nucleic acids and amino acids have aromatic structures, which play a crucial role in their function.
- Methylbenzene: Methylbenzene, an aromatic hydrocarbon, is used as a solvent in model glues.
- Naphthalene: Naphthalene is a key ingredient in the production of mothballs.
- Phenanthrene: Phenanthrene is used in the synthesis of drugs, dyes, and explosives.
- Trinitrotoluene (TNT): TNT is a widely used aromatic hydrocarbon for explosive purposes.
- Plastics and Petrochemical Industries: Aromatic hydrocarbons are extensively used in the plastics and petrochemical industries.
Polycyclic Aromatic Hydrocarbons
- Polycyclic aromatic hydrocarbons (PAHs) are made up of multiple fused aromatic rings. They are commonly found in substances like coal, tar, oil, and certain cooked foods, including smoked fish and burnt toast.
- Naphthalene is a well-known example of a polycyclic hydrocarbon. PAHs are recognized as environmental pollutants due to their harmful effects.
- Other examples of aromatic hydrocarbons include toluene, phenanthrene, trinitrotoluene, and o-dihydroxybenzene.
|
114 videos|263 docs|74 tests
|
FAQs on Aromatic Hydrocarbons: Nomenclature, Properties, Reactions, Uses & Polycyclic Aromatic Hydrocarbons - Chemistry Class 11 - NEET
| 1. What are aromatic hydrocarbons and how do they differ from aliphatic hydrocarbons? |  |
| 2. How are aromatic hydrocarbons named according to IUPAC nomenclature? |  |
| 3. What are the key properties of aromatic hydrocarbons? |  |
| 4. What are some common reactions of aromatic hydrocarbons? |  |
| 5. What are polycyclic aromatic hydrocarbons (PAHs) and why are they significant? |  |






















